LIBERVANT- diazepam film
LIBERVANT by
Drug Labeling and Warnings
LIBERVANT by is a Prescription medication manufactured, distributed, or labeled by AQUESTIVE THERAPEUTICS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LIBERVANTTM safely and effectively. See full prescribing information for LIBERVANT.
LIBERVANT (diazepam) buccal film, C-IV
Initial U.S. Approval: 1963
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; AND DEPENDENCE AND WITHDRAWAL REACTIONS
See full prescribing information for complete boxed warning.
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. (5.1, 7.1)
- LIBERVANT is approved for use in pediatric patients 2 to 5 years of age. The unapproved use of LIBERVANT exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing LIBERVANT and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. (5.2)
- Although LIBERVANT is indicated only for intermittent use (1, 2), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of LIBERVANT may precipitate acute withdrawal reactions, which can be life-threatening. For patients using LIBERVANT more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue LIBERVANT. (5.3)
INDICATIONS AND USAGE
LIBERVANT is a benzodiazepine indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 2 to 5 years of age. (1)
DOSAGE AND ADMINISTRATION
The recommended dose of LIBERVANT is dependent on the patient’s weight. See dosing table for specific recommendations. (2.2)
DOSAGE FORMS AND STRENGTHS
Buccal film: 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg (3)
WARNINGS AND PRECAUTIONS
- CNS Depression: Monitor for central nervous system (CNS) depression. Risk may be increased with concomitant use of alcohol or other CNS depressants. (5.2,5.4, 7.2)
- Suicidal Behavior and Ideation: Antiepileptic drugs increase the risk of suicidal thoughts and behavior. (5.5)
- Glaucoma: Can increase intraocular pressure; use in patients with open-angle glaucoma only if receiving appropriate therapy. (4, 5.6)
ADVERSE REACTIONS
- The most common adverse reactions (> 4%) were somnolence and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Aquestive Therapeutics, Inc. at 1-877-394-5045 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION and DEPENDENCE AND WITHDRAWAL REACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Instructions Prior to Dosing
2.2 Dosage Information
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 Abuse, Misuse, and Addiction
5.3 Dependence and Withdrawal ReactionsAfter Use of LIBERVANT More Frequently Than Recommended
5.4 Central Nervous System (CNS) Depression
5.5 Suicidal Behavior and Ideation
5.6 Glaucoma
5.7 Neonatal Sedation and Withdrawal Syndrome
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Use of Benzodiazepines and Opioids
7.2 CNS Depressants and Alcohol
7.3 Effect of Other Drugs on LIBERVANT Metabolism
7.4 Effect of LIBERVANT on the Metabolism of Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Compromised Respiratory Function
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- LIBERVANT is approved for use in pediatric patients 2 to 5 years of age. The unapproved use of LIBERVANT exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although LIBERVANT is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of LIBERVANT may precipitate acute withdrawal reactions, which can be life-threatening. For patients using LIBERVANT more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue LIBERVANT [see Warnings and Precautions (5.3)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Instructions Prior to Dosing
Prior to treatment, healthcare professionals should instruct the individual administering LIBERVANT on how to identify seizure clusters and use the product appropriately [see Dosage and Administration (2.3) and Patient Counseling Information (17)].
2.2 Dosage Information
The recommended dose of LIBERVANT is dependent on the patient’s weight and is provided in Table 1.
Table 1: Recommended Dosage for Pediatric Patients 2 to 5 Years of Age Weight Libervant Dose 6 kg to 10 kg 5 mg 11 kg to 15 kg 7.5 mg 16 kg to 20 kg 10 mg 21 kg to 25 kg 12.5 mg 26 kg to 30 kg 15 mg Second Dose (if needed): A second dose, when required, may be administered at least 4 hours after the first dose.
Maximum Dosage and Treatment Frequency: Do not use more than 2 doses of LIBERVANT to treat a single episode.
Do not use LIBERVANT to treat more than one episode every five days or more than five episodes per month.
2.3 Important Administration Instructions
Caregivers should be counseled to read carefully the “Instructions for Use” for complete directions on how to properly administer LIBERVANT.
LIBERVANT is a rectangular green buccal film that dissolves when applied on the inside of the mouth on top of the surface of the cheek. Do not split LIBERVANT, the entire dose should be applied and allowed to dissolve [see Dosage and Administration (2.1)]. Do not administer LIBERVANT buccal film with liquids.
LIBERVANT dosing may be administered without regard to food [see Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
LIBERVANT is contraindicated in patients with:
- Hypersensitivity to diazepam
- Acute narrow-angle glaucoma [see Warnings and Precautions (5.6)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including LIBERVANT, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe LIBERVANT concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise caregivers about the risks of respiratory depression and sedation when LIBERVANT is used with opioids [see Drug Interactions (7.1)].
5.2 Abuse, Misuse, and Addiction
LIBERVANT is approved for use in pediatric patients 2 to 5 years of age. The unapproved use of LIBERVANT exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)].
5.3 Dependence and Withdrawal ReactionsAfter Use of LIBERVANT More Frequently Than Recommended
For patients using LIBERVANT more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue LIBERVANT (a patient-specific plan should be used to taper the dose).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines may lead to clinically significant physical dependence. Although LIBERVANT is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of LIBERVANT, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) [see Drug Abuse and Dependence (9.3)].
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Drug Abuse and Dependence (9.3)].
5.4 Central Nervous System (CNS) Depression
Because LIBERVANT produces CNS depression, patients receiving this drug, who are otherwise capable and qualified to do so should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery, driving a motor vehicle, or riding a bicycle, until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits.
Although LIBERVANT is indicated for use solely on an intermittent basis, the potential for a synergistic CNS-depressant effect when used simultaneously with alcohol or other CNS depressants must be considered by the prescriber, and appropriate recommendations made to the patient and/or caregiver.
5.5 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including LIBERVANT, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed. The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
Table 2: Risk by Indication for Antiepileptic Drugs in the Pooled Analysis Indication Placebo Patients with Events per 1000 Patients Drug Patients with Events per 1000 Patients Relative Risk: Incidence of Drug Events in Drug Patients / Incidence in Placebo Patients Risk Difference: Additional Drug Patients with Events per 1000 Patients Epilepsy 1.0 3.4 3.5 2.4 Psychiatric 5.7 8.5 1.5 2.9 Other 1.0 1.8 1.9 0.9 Total 2.4 4.3 1.8 1.9 The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing LIBERVANT, or any other AED, must balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.6 Glaucoma
Benzodiazepines, including LIBERVANT, can increase intraocular pressure in patients with glaucoma. LIBERVANT may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. LIBERVANT is contraindicated in patients with narrow-angle glaucoma.
5.7 Neonatal Sedation and Withdrawal Syndrome
LIBERVANT is not approved for use in adolescents and adults. Unapproved use of LIBERVANT in adolescents and adults late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to LIBERVANT during pregnancy or labor for signs of sedation and monitor neonates exposed to LIBERVANT during pregnancy for signs of withdrawal; manage these neonates accordingly.
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
LIBERVANT is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs, including LIBERVANT. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (LIBERVANT contains 3.96 to 11.87 mg of benzyl alcohol per buccal film) [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling.
- Risk of Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- Dependence and Withdrawal Reactions After Use of LIBERVANT More Frequently Than Recommended [see Warnings and Precautions (5.3)]
- CNS depression [see Warnings and Precautions (5.4)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.5)]
- Glaucoma [see Warnings and Precautions (5.6)]
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.7)]
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of LIBERVANT is supported by clinical trials using diazepam rectal gel; pharmacokinetic studies of LIBERVANT in healthy subjects and epilepsy patients; and an open-label long-term safety and tolerability study of LIBERVANT in epilepsy patients.
Diazepam Rectal Gel
In studies previously conducted with diazepam rectal gel, adverse event data were collected from double-blind, placebo-controlled studies and open-label studies. The majority of adverse events were mild to moderate in severity and transient in nature.
Two patients who received diazepam rectal gel died seven to 15 weeks following treatment; neither of these deaths was deemed related to diazepam rectal gel.
The most frequent adverse reactions (at least 5%) in the two double-blind, placebo-controlled studies were somnolence and headache. Table 3 lists adverse reactions that occurred in greater than 1% of patients enrolled in parallel-group, placebo-controlled trials and were numerically more common in the diazepam rectal gel group than placebo. Adverse reactions were usually mild or moderate in intensity.
Approximately 1.4% of the 573 patients who received diazepam rectal gel in clinical trials of epilepsy discontinued treatment because of an adverse event. The adverse reaction most frequently associated with discontinuation (occurring in three patients) was somnolence. Other adverse reactions most commonly associated with discontinuation and occurring in two patients were hypoventilation and rash. Adverse reactions associated with discontinuation occurring in one patient were asthenia, hyperkinesia, incoordination, vasodilatation, and urticaria.
In the two double-blind, placebo-controlled, parallel-group studies [see Clinical Studies (14)], the proportion of patients who discontinued treatment because of adverse events was 2% for the group treated with diazepam rectal gel, versus 2% for the placebo group. In the diazepam rectal gel group, one patient discontinued because of rash and one patient discontinued because of lethargy.
Table 3: Adverse Reactions That Occurred in Greater Than 1% Of Patients in Parallel-Group, Placebo-Controlled Trials with Diazepam Rectal Gel and Were More Common Than in the Placebo Group Adverse Reaction Diazepam Rectal Gel
N = 101
%Placebo
N = 104
%Somnolence 23 8 Headache 5 4 Diarrhea 4 <1 Ataxia 3 <1 Dizziness 3 2 Euphoria 3 0 Incoordination 3 0 Rash 3 0 Asthma 2 0 Vasodilatation 2 0 LIBERVANT (Diazepam Buccal Film)
Clinical studies, which included patients with epilepsy 2 to 5 years of age, were conducted to support the safety and tolerability of LIBERVANT for the treatment of acute repetitive seizures. A total of 197 patients received LIBERVANT, of whom 107 received LIBERVANT for at least 6 months, and 48 for at least 1 year. The adverse reactions reported in these studies were similar to those seen in efficacy trials of diazepam rectal gel.
Other Adverse Reactions
Diazepam rectal gel was administered to 573 patients with epilepsy during all clinical trials, only some of which were placebo controlled. All of the events listed below occurred in at least 1% of the 573 individuals exposed to diazepam rectal gel.
Body as a Whole: Asthenia
Cardiovascular: Hypotension, vasodilatation
Nervous: Agitation, confusion, convulsion, dysarthria, emotional lability, speech disorder, thinking abnormal, vertigo
Respiratory: Hiccup
The following infrequent adverse events have been reported previously with diazepam use: depression, slurred speech, syncope, constipation, changes in libido, urinary retention, bradycardia, cardiovascular collapse, nystagmus, urticaria, neutropenia, and jaundice.
Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported with other diazepam products. If these events occur with the use of LIBERVANT, the prescriber should consider discontinuation of use.
- Risk of Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Use of Benzodiazepines and Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids and follow patients closely for respiratory depression and sedation [see Warnings and Precautions (5.1)].
7.2 CNS Depressants and Alcohol
Coadministration of other CNS depressants (e.g., valproate) or consumption of alcohol may potentiate the CNS-depressant effects of diazepam [see Warnings and Precautions (5.2)].
7.3 Effect of Other Drugs on LIBERVANT Metabolism
Potential interactions may occur when diazepam is given concurrently with agents that affect CYP2C19 and CYP3A4 activity.
Inhibitors of CYP2C19 and CYP3A4
Inhibitors of CYP2C19 (e.g., cimetidine, quinidine, and tranylcypromine) and CYP3A4 (e.g., ketoconazole, troleandomycin, and clotrimazole) could decrease the rate of diazepam elimination; therefore, adverse reactions to LIBERVANT may be increased.
Inducers of CYP2C19 and CYP3A4
Inducers of CYP2C19 (e.g., rifampin) and CYP3A4 (e.g., carbamazepine, phenytoin, dexamethasone, and phenobarbital) could increase the rate of diazepam elimination; therefore, efficacy of LIBERVANT may be decreased.
7.4 Effect of LIBERVANT on the Metabolism of Other Drugs
Diazepam is a substrate for CYP2C19 and CYP3A4; therefore, it is possible that LIBERVANT may interfere with the metabolism of drugs which are substrates for CYP2C19, (e.g. omeprazole, propranolol, and imipramine) and CYP3A4 (e.g. cyclosporine, paclitaxel, theophylline, and warfarin) leading to a potential drug-drug interaction [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
LIBERVANT is indicated for treatment in patients 2 to 5 years of age [see Indications and Usage (1)]. LIBERVANT is not approved for use in adolescents and adults.
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as LIBERVANT, during pregnancy. Healthcare providers are encouraged to recommend that pregnant women who are taking LIBERVANT during pregnancy enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [see Warnings and Precautions (5.7) and Clinical Considerations]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (seeHuman Data).
In animal studies, administration of diazepam during the organogenesis period of pregnancy resulted in increased incidences of fetal malformations at doses greater than those used clinically. Data for diazepam and other benzodiazepines suggest the possibility of increased neuronal cell death and long-term effects on neurobehavioral and immunological function based on findings in animals following prenatal or early postnatal exposure at clinically relevant doses (see Animal Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to LIBERVANT during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to LIBERVANT during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.7)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects.
Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
Diazepam has been shown to produce increased incidences of fetal malformations in mice and hamsters when given orally at single doses of 100 mg/kg or greater (approximately 13 times a human dose of 0.6 mg/kg/day or greater on a mg/m2 basis). Cleft palate and exencephaly are the most common and consistently reported malformations produced in these species by administration of high, maternally toxic doses of diazepam during organogenesis.
In published animal studies, administration of benzodiazepines or other drugs that enhance GABAergic neurotransmission to neonatal rats has been reported to result in widespread apoptotic neurodegeneration in the developing brain at plasma concentrations relevant for seizure control in humans. The window of vulnerability to these changes in rats (postnatal days 0-14) includes a period of brain development corresponding to that taking place during the third trimester of pregnancy in humans.
8.2 Lactation
LIBERVANT is indicated for treatment in patients 2 to 5 years of age [see Indications and Usage (1)]. LIBERVANT is not approved for use in adolescents and adults.
Risk Summary
Diazepam is excreted in human milk.
There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data to assess the effects of diazepam and/or its active metabolite(s) on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for LIBERVANT and any potential adverse effects on the breastfed infant from LIBERVANT or from the underlying maternal condition.
Clinical Considerations
Infants exposed to LIBERVANT through breast milk should be monitored for sedation, poor feeding, and poor weight gain.
8.4 Pediatric Use
Safety and effectiveness of LIBERVANT have been established in pediatric patients 2 to 5 years of age. Use of LIBERVANT in this age group is supported by evidence from adequate and well-controlled studies of diazepam rectal gel in adult and pediatric patients, adult bioavailability studies comparing LIBERVANT with diazepam rectal gel, pediatric and adult LIBERVANT pharmacokinetic data, and an open-label safety study of LIBERVANT including patients 2 years to 5 years of age [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Safety and effectiveness of LIBERVANT in pediatric patients below the age of 2 and above the age of 5 have not been established.
LIBERVANT is not approved for use in neonates or infants.
- Prolonged CNS depression has been observed in neonates treated with diazepam.
- Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and low-birth-weight infants in the neonatal intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth-weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (LIBERVANT contains 3.96 to 11.87 mg of benzyl alcohol per buccal film [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
LIBERVANT is indicated for treatment in patients 2 to 5 years of age [see Indications and Usage (1)]. LIBERVANT is not approved for use in adults.
Clinical studies of LIBERVANT did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
A study of single dose IV administration of diazepam (0.1 mg/kg) indicates that the elimination half-life of diazepam increases linearly with age, ranging from about 15 hours at 18 years (healthy young adults) to about 100 hours at 95 years (healthy elderly) with a corresponding decrease in clearance of free diazepam.
If used in elderly patients, LIBERVANT should be used with caution because of an increase in half life with a corresponding decrease in the clearance of free diazepam [see Clinical Pharmacology (12.3)]. It is also recommended that the dosage be decreased to reduce the likelihood of ataxia or oversedation.
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
LIBERVANT is a benzodiazepine and a CNS depressant with a potential for abuse and addiction with unapproved use in adolescents and adults. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
9.3 Dependence
Physical Dependence After Use of LIBERVANT More Frequently Than Recommended
LIBERVANT may produce physical dependence if used more frequently than recommended. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Although LIBERVANT is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)].
For patients using LIBERVANT more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue LIBERVANT [seeWarnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to LIBERVANT may develop after use more frequently than recommended. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of benzodiazepines may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
It is recommended that patients be treated with LIBERVANT no more frequently than one episode every five days and no more than five episodes per month.
LIBERVANT is not recommended for chronic, daily use as an anticonvulsant. Chronic daily use of diazepam may increase the frequency and/or severity of tonic-clonic seizures, requiring an increase in the dosage of standard anticonvulsant medication. In such cases, abrupt withdrawal of chronic diazepam may also be associated with a temporary increase in the frequency and/or severity of seizures.
-
10 OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
-
11 DESCRIPTION
Diazepam, the active ingredient of LIBERVANT, is a benzodiazepine anticonvulsant with the chemical name 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one; its empirical formula is C16H13ClN2O and its molecular weight is 284.7 g/mol. It is an odorless, white to practically white crystalline powder with a pKa of 3.4 and a partition coefficient of 2.9. It is practically insoluble in water and soluble in 96% ethanol, chloroform, and alcohol. The melting range for diazepam is 131°C to 135°C.The structural formula is as follows:
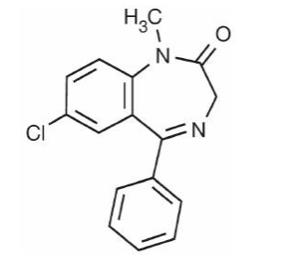
LIBERVANT is a buccal film that contains the active ingredient diazepam. Each film strip contains 5, 7.5, 10, 12.5, or 15mg of diazepam and the following inactive ingredients: benzyl alcohol, clove oil, EDTA disodium salt, FD&C Green #3, glycerol monooleate, hypromellose, peppermint oil, polyethylene oxide, polyvinylpyrrolidone, sodium phosphate, sucralose, vanillin, xanthan gum, water, and white ink.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for diazepam is not fully understood, but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
12.2 Pharmacodynamics
The effects of diazepam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
12.3 Pharmacokinetics
The pharmacokinetics of diazepam and desmethyldiazepam following administration of LIBERVANT were investigated in adult healthy subjects and in pediatric and adult patients with epilepsy. The pharmacokinetics of diazepam were linear and dose-proportional in the recommended dose range [see Dosage and Administration (2.2)]. In pediatric patients 2 to 5 years of age, median maximum plasma concentration (Cmax) and median area under the plasma concentration curve for 4 hours after dosing (AUC0-4) of diazepam are approximately 2- to 3-times greater than in adults. The higher Cmax and higher AUC0-4 in pediatric patients 2 to 5 years of age are expected to provide adequate therapeutic exposures under both fed and fasted states.
Absorption
Following single doses of LIBERVANT administered to healthy adults under fasting conditions, diazepam peak concentration (Cmax) was reached in approximately 1 hour.
In pharmacokinetic studies of patients with epilepsy, pharmacokinetic parameters were similar between seizure state and non-seizure state.
Effect of Food
The pharmacokinetics of LIBERVANT were characterized under fasting, moderate fat, and high fat fed conditions in three clinical studies in adults. Under fed conditions a 33%-47% decrease in Cmax, but no significant change in AUC, was observed relative to fasting state. The recommended dosage of LIBERVANT [see Dosage and Administration (2.3)] considers the impact of food on pharmacokinetics of diazepam. As such, LIBERVANT may be administered without regard to food.
Distribution
The volume of distribution of diazepam was calculated to be approximately 1.46 L/kg. Both diazepam and its major active metabolite, desmethyldiazepam, bind extensively to plasma proteins (95%-98%).
Elimination
Metabolism
It has been reported in the literature that diazepam is extensively metabolized to one major active metabolite (desmethyldiazepam) and two minor active metabolites, 3-hydroxydiazepam (temazepam) and 3-hydroxy-N-diazepam (oxazepam) in plasma. At therapeutic doses, desmethyldiazepam is found in plasma at concentrations equivalent to those of diazepam while oxazepam and temazepam are not usually detectable. The metabolism of diazepam is primarily hepatic and involves demethylation (involving primarily CYP2C19 and CYP3A4) and 3-hydroxylation (involving primarily CYP3A4), followed by glucuronidation. The marked inter-individual variability in the clearance of diazepam reported in the literature is probably attributable to variability of CYP2C19 (which is known to exhibit genetic polymorphism; about 3-5% of Caucasians have little or no activity and are “poor metabolizers”) and CYP3A4. No inhibition was demonstrated in the presence of inhibitors selective for CYP2A6, CYP2C9, CYP2D6, CYP2E1, or CYP1A2, indicating that these enzymes are not significantly involved in metabolism of diazepam.
Excretion
The mean elimination half-lives of diazepam and desmethyldiazepam were found to be approximately 86 hours and 147 hours, respectively.
Specific Populations
Pediatric Patients
Literature reviews indicate that following IV administration (0.33 mg/kg), the half-life of diazepam in pediatric patients 2 to 5 years of age is approximately 15 to 21 hours.
Patients with Renal Impairment
The pharmacokinetics of diazepam have not been studied in subjects with renal impairment.
Patients with Hepatic Impairment
No pharmacokinetic studies were conducted with LIBERVANT in subjects with hepatic impairment. Literature review indicates that following administration of 0.1 to 0.15 mg/kg of diazepam intravenously, the half-life of diazepam was prolonged by two to five-fold in subjects with alcoholic cirrhosis (n=24) compared to age-matched control subjects (n=37) with a corresponding decrease in clearance by half. However, the exact degree of hepatic impairment in these subjects was not characterized in this literature.
Effect of Gender, Race, and Cigarette Smoking
No targeted pharmacokinetic studies have been conducted to evaluate the effect of gender, race, and cigarette smoking on the pharmacokinetics of diazepam. However, covariate analysis of a population of treated patients following administration of diazepam rectal gel, indicated that neither gender nor cigarette smoking had any effect on the pharmacokinetics of diazepam.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In studies in which mice and rats were administered diazepam in the diet at a dose of 75 mg/kg/day (approximately 10 and 20 times, respectively, a human dose of 0.6 mg/kg/day on a mg/m2 basis) for 80 and 104 weeks, respectively, an increased incidence of liver tumors was observed in males of both species.
Mutagenesis
The data currently available are inadequate to determine the mutagenic potential of diazepam.
Impairment of Fertility
Reproduction studies of diazepam in rats showed decreases in the number of pregnancies and in the number of surviving offspring following administration of an oral dose of 100 mg/kg/day (approximately 27 times a human dose of 0.6 mg/kg/day on a mg/m2 basis) prior to and during mating and throughout gestation and lactation. No adverse effects on fertility or offspring viability were noted at a dose of 80 mg/kg/day (approximately 22 times a human dose of 0.6 mg/kg/day on a mg/m2 basis).
-
14 CLINICAL STUDIES
Safety and effectiveness of LIBERVANT in pediatric patients 2 to 5 years of age are supported by evidence from adequate and well-controlled studies of diazepam rectal gel in adult and pediatric patients, adult bioavailability studies comparing LIBERVANT with diazepam rectal gel, adult and pediatric LIBERVANT pharmacokinetic data, and an open-label safety study of LIBERVANT including patients 2 years to 5 years of age [see Clinical Pharmacology (12.3)].
The effectiveness of diazepam rectal gel has been established in two adequate and well-controlled clinical studies in pediatric patients 2 years of age and older and adults exhibiting intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern.
A randomized, double-blind study compared sequential doses of diazepam rectal gel and placebo in 91 patients (47 pediatric patients 2 years of age and older, 44 adults) exhibiting the appropriate seizure profile. The first dose was given at the onset of an identified episode. Pediatric patients 2 years of age and older were dosed again 4 hours after the first dose and were observed for a total of 12 hours. Adults were dosed at 4 and 12 hours after the first dose and were observed for a total of 24 hours. Primary outcomes for this study were seizure frequency during the period of observation and a global assessment that took into account the severity and nature of the seizures as well as their frequency.
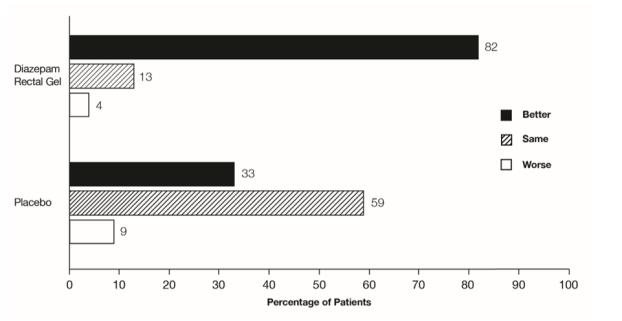
The median seizure frequency for the diazepam rectal gel treated group was zero seizures per hour, compared to a median seizure frequency of 0.3 seizures per hour for the placebo group, a difference that was statistically significant (p <0.0001). All three categories of the global assessment (seizure frequency, seizure severity, and “overall”) were also found to be statistically significant in favor of diazepam rectal gel (p < 0.0001). The following histogram displays the results for the “overall” category of the global assessment.
Figure 1: Caregiver Overall Global Assessment of the Efficacy of Diazepam Rectal Gel
Patients treated with diazepam rectal gel experienced prolonged time-to-next-seizure compared to placebo (p = 0.0002) as shown in the following graph.
Figure 2: Kaplan-Meier Survival Analysis of Time-to-Next-Seizure - First Study
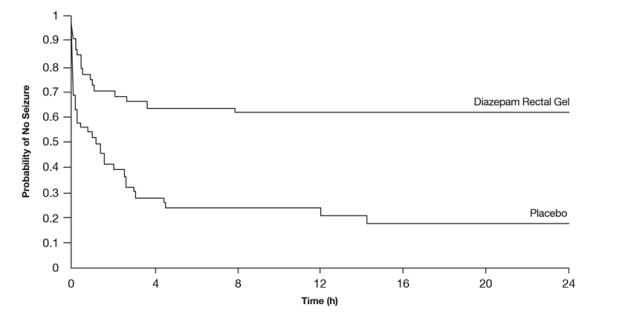
In addition, 62% of patients treated with diazepam rectal gel were seizure-free during the observation period compared to 20% of placebo patients.
Analysis of response by gender and age revealed no substantial differences between treatment in either of these subgroups. Analysis of response by race was considered unreliable, due to the small percentage of non-Caucasians.
A second double-blind study compared single doses of diazepam rectal gel and placebo in 114 patients (53 pediatric patients 2 years of age and older, 61 adults). The dose was given at the onset of the identified episode and patients were observed for a total of 12 hours. The primary outcome in this study was seizure frequency. The median seizure frequency for the diazepam rectal gel-treated group was 0 seizures per 12 hours, compared to a median seizure frequency of 2.0 seizures per 12 hours for the placebo group, a difference that was statistically significant (p < 0.03). Patients treated with diazepam rectal gel experienced prolonged time-to-next-seizure compared to placebo (p = 0.0072) as shown in Figure 3.
Figure 3: Kaplan-Meier Survival Analysis of Time-to-Next-Seizure - Second Study
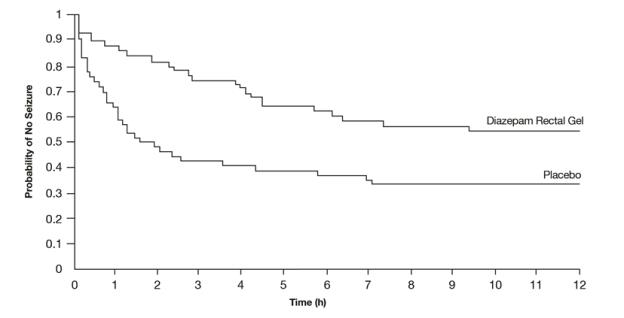
In addition, 55% of patients treated with diazepam rectal gel were seizure-free during the observation period compared to 34% of patients receiving placebo. Overall, caregivers judged diazepam rectal gel to be more effective than placebo (p = 0.018), based on a 10 cm visual analog scale. In addition, investigators also evaluated the effectiveness of diazepam rectal gel and judged diazepam rectal gel to be more effective than placebo (p < 0.001).
An analysis of response by gender revealed a statistically significant difference between treatments in females but not in males in this study, and the difference between the 2 genders in response to the treatments reached borderline statistical significance. Analysis of response by race was considered unreliable, due to the small percentage of non-Caucasians.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
LIBERVANT (diazepam buccal film) is supplied as five strengths of a rectangular green film imprinted in white ink according to their respective strengths and packaged in individual child-resistant polyester/foil laminated pouches (see Table 4).
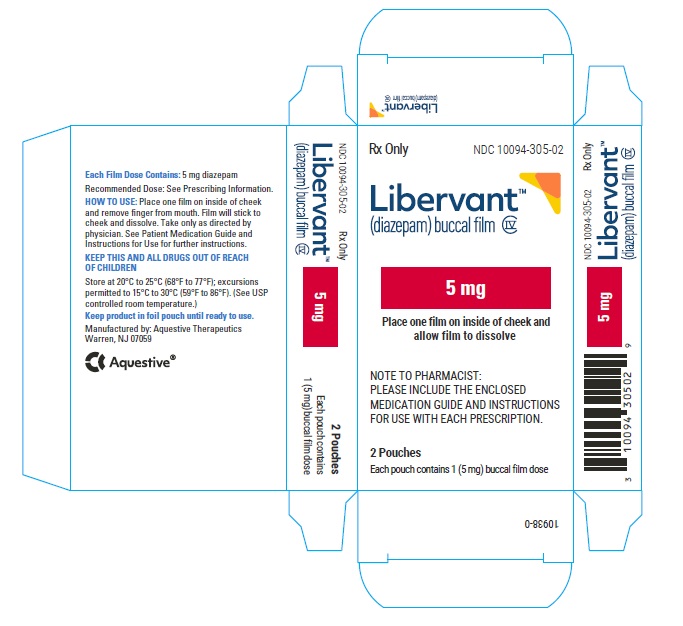
Table 4: Available Packaging Configurations Description Contents NDC 5 mg carton 5 mg film strip imprinted with D5 NDC: 10094-305-01 (Individual pouch)
NDC: 10094-305-02 (Carton of 2 pouches)
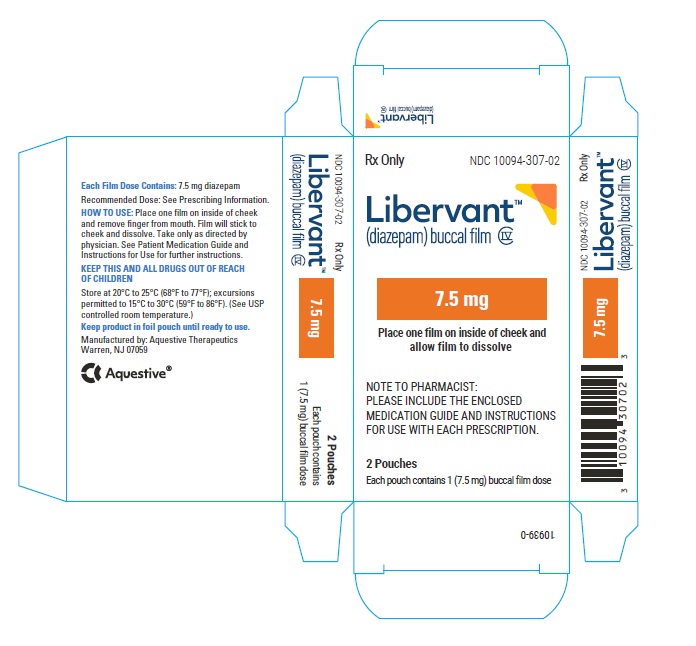
7.5 mg carton 7.5 mg film strip imprinted with D7●5 NDC: 10094-307-01 (Individual pouch)
NDC: 10094-307-02 (Carton of 2 pouches)
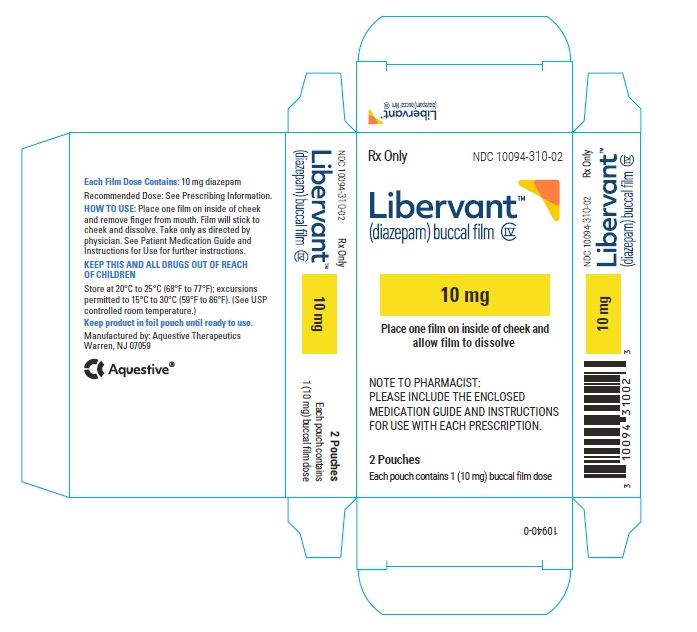
10 mg carton 10 mg film strip imprinted with D10 NDC: 10094-310-01 (Individual pouch)
NDC10094-310-02 (Carton of 2 pouches)
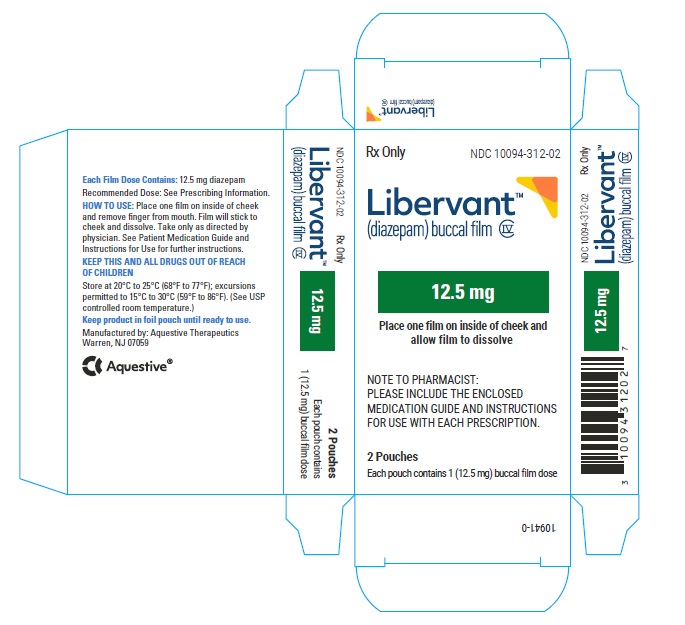
12.5 mg carton 12.5 mg film strip imprinted with D12●5 NDC: 10094-312-01 (Individual pouch)
NDC: 10094-312-02 (Carton of 2 pouches)
15 mg carton 15 mg film strip imprinted with D15 NDC: 10094-315-01 (Individual pouch)
NDC: 10094-315-02 (Carton of 2 pouches)
-
17 PATIENT COUNSELING INFORMATION
Advise the caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risks from Concomitant Use with Opioids
Inform caregivers that concomitant use of benzodiazepines, including LIBERVANT, and opioids may result in profound sedation, respiratory depression, coma, and death and not to use such drugs concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Abuse, Misuse, and Addiction
Inform caregivers that the use of LIBERVANT more frequently than recommended, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform caregivers about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2) and Drug Abuse and Dependence (9.2)].
Withdrawal Reactions
Inform caregivers that the use of LIBERVANT more frequently than recommended may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of LIBERVANT may precipitate acute withdrawal reactions, which can be life-threatening. Inform caregivers that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Warnings and Precautions (5.3) and Drug Abuse and Dependence (9.2)].
Important Treatment Instructions
Instruct caregivers on what is and is not an intermittent and stereotypic episode of increased seizure activity (i.e., seizure cluster) that is appropriate for treatment, and the timing of administration in relation to the onset of the episode.
Instruct caregivers on what to observe following administration, and what would constitute an outcome requiring immediate medical attention.
Instruct caregivers not to administer a second dose of LIBERVANT if they are concerned by the patient’s breathing, the patient requires emergency rescue treatment with assisted breathing or intubation, or there is excessive sedation [see Use in Specific Populations (8.6)].
Advise caregivers on how frequently they can treat successive seizure cluster episodes over time.
CNS Depression
Advise caregivers to check with their healthcare provider before LIBERVANT is taken with other CNS depressants, such as other benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or alcohol [see Warnings and Precautions (5.4)].If applicable, caution patients and caregivers about operating hazardous machinery, including driving a motor vehicle, or riding a bicycle, until they are reasonably certain that LIBERVANT does not affect them adversely (e.g., impair judgment, thinking or motor skills).
Suicidal Thinking and Behavior
Counsel patients, their caregivers, and their families that AEDs, including LIBERVANT, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Caregivers should report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.5)].Proper Administration
Discuss the steps involved in the administration of LIBERVANT with the caregiver. The steps are described in the Medication Guide and Instruction for Use.
Manufactured by:
Aquestive Therapeutics
Warren, NJ -
MEDICATION GUIDE
MEDICATION GUIDE
LIBERVANT™(lih-ber-vant)
(diazepam)
buccal film, C-IVWhat is the most important information I should know about LIBERVANT?
-
LIBERVANT is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death. Get emergency help right away if any of the following happens:
◦ shallow or slowed breathing,
◦ breathing stops (which may lead to the heart stopping),
◦ excessive sleepiness (sedation).
-
Risk of abuse, misuse, and addiction. LIBERVANT is used in children 2 to 5 years of age. The unapproved use of LIBERVANT has a risk for abuse, misuse, and addiction, which can lead to overdose and serious side effects including coma and death.
◦ Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including diazepam (the active ingredient in LIBERVANT). These serious side effects may also includedelirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your child’s healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
◦ Your child can develop an addiction even if your child takes LIBERVANT as prescribed by your child’s healthcare provider.
◦ Give LIBERVANT exactly as your child’s healthcare provider prescribed.
◦ Do not share LIBERVANT with other people.
◦ Keep LIBERVANT in a safe place and away from children. -
Physical dependence and withdrawal reactions. LIBERVANT is intended for use if needed in order to treat higher than usual seizure activity. Benzodiazepines, including LIBERVANT, can cause physical dependence and withdrawal reactions, especially if used daily. LIBERVANT is not intended for daily use.
◦ Do not suddenly stop giving LIBERVANT to your child without talking to your child’s healthcare provider. Stopping LIBERVANT suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures that will not stop (status epilepticus), sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, homicidal thoughts, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your child’s healthcare provider or go to the nearest hospital emergency room right away if your child gets any of these symptoms.
◦ Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months including,anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning, or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
◦Physical dependence is not the same as drug addiction. Your child’s healthcare provider can tell you more about the differences between physical dependence and drug addiction.
◦ Do not give your child more LIBERVANT than prescribed or give LIBERVANT more often than prescribed.
-
LIBERVANT can make your child sleepy or dizzy and can slow your child’s thinking and motor skills.
◦ Do not allow your child to drive a motor vehicle, operate machinery, or ride a bicycle until you know how LIBERVANT affects your child.
◦ Do not give other drugs that may make your child sleepy or dizzy while taking LIBERVANT without first talking to your child’s healthcare provider. When taken with drugs that cause sleepiness or dizziness, LIBERVANT may make your child’s sleepiness or dizziness much worse.
Call a healthcare provider right away if your child has any of these symptoms, especially if they are new, worse, or worry you:- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety or irritability
- feeling agitated or restless
- an extreme increase in activity and talking (mania)
- trouble sleeping (insomnia)
- acting aggressive, being angry or violent
- new or worse panic attacks
- other unusual changes in behavior or mood
- acting on dangerous impulses
How can I watch for early symptoms of suicidal thoughts or actions?
- Pay attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your child’s healthcare provider as scheduled.
What is LIBERVANT?
- LIBERVANT is a prescription medicine used for the short-term treatment of seizure clusters (also known as “acute repetitive seizures”) that are different from a person’s usual seizure pattern in people 2 to 5 years of age.
Do not give LIBERVANT to your child if your child:
- is allergic to diazepam or any of the ingredients in LIBERVANT. See the end of this Medication Guide for a complete list of ingredients in LIBERVANT.
- has an eye problem called acute narrow angle glaucoma.
Before you give LIBERVANT, tell your child’s healthcare provider about all of your child’s medical conditions, including if your child:
- has a history of depression, mood problems, or suicidal thoughts or behavior.
- has liver or kidney problems.
- has asthma, emphysema, bronchitis, chronic obstructive pulmonary disease, or other breathing problems.
How should I give LIBERVANT?
- Read the Instructions for Use that comes with this Medication Guide for detailed information about the right way to give LIBERVANT.
- Give LIBERVANT exactly as your child’s healthcare provider tells you to.
- Do not change your child’s dosage unless your child’s healthcare provider tells you to change it.
- To give LIBERVANT:
◦ You as the caregiver must be able to tell the difference between cluster seizures and ordinary seizures.
◦ You as the caregiver must be comfortable in your ability to give LIBERVANT as instructed by your child’s healthcare provider and as instructed in the Instructions for Use at the end of this Medication Guide.
◦ You as the caregiver must understand your child’s healthcare provider’s instructions about when to use LIBERVANT. - Your child’s healthcare provider will tell you:
◦ what seizure clusters are,
◦ exactly how much LIBERVANT to give to your child,
◦ when to give LIBERVANT,
◦ how to give LIBERVANT,
◦ what to do after you give LIBERVANT if the seizures do not stop or there is a change in breathing, behavior, or condition that worries you. - Your child’s healthcare provider should show you how to give LIBERVANT the right way.
- You should carry LIBERVANT with you in case you need to give it to treat your child’s seizure clusters.
- Before a seizure cluster happens, family members, caregivers, and other people who may have to give LIBERVANT should know where you keep your LIBERVANT and how to give LIBERVANT.
- Each LIBERVANT comes in a sealed foil pouch. Do not open the foil pouch until you are ready to use it.
- Do not split LIBERVANT. The entire film should be placed on the inside of the cheek.
- Allow LIBERVANT to dissolve. Do not chew or swallow the film.
- Do not take with liquids.
- LIBERVANT can be taken with or without food.
-
What should I do after LIBERVANT has been given:
◦ Note the time LIBERVANT was given and changes in the resting breathing rate.
◦ Your child’s healthcare provider may prescribe a second dose of LIBERVANT.
◦ If a second dose is needed, it may be given at least 4 hours after the first dose of LIBERVANT film is given.
◦ Do not give more than 2 doses of LIBERVANT to treat a seizure cluster.
◦ Do not give a second dose of LIBERVANT if:
▪ an emergency rescue treatment with breathing help is needed,
▪ or there is more sleepiness than normal.
Call for emergency medical help if any of the following happens:
◦ seizure behavior is different from other episodes the child has had.
◦ you are alarmed by how often the seizure happens, by how severe it is, by how long it lasts, or seizure is alarming.
◦ the child has unusual coloring or breathing.
-
Do not use LIBERVANT for:
◦ more than 1 seizure cluster episode every 5 days and
◦ more than 5 episodes each month. - Talk to your child’s healthcare provider about slowly stopping LIBERVANT to avoid withdrawal symptoms.
- If you give too much LIBERVANT, call your child’s healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of LIBERVANT?
LIBERVANT may cause serious side effects, including:
- See “What is the most important information I should know about LIBERVANT?”
- sleepiness
- headache
These are not all the possible side effects of LIBERVANT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store LIBERVANT? - Store LIBERVANT at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep LIBERVANT in the foil pouch until ready to use.
- Keep LIBERVANT and all medicines out of the reach of children.
General information about the safe and effective use of LIBERVANT.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LIBERVANT for a condition for which it was not prescribed. Do not give LIBERVANT to other people, even if they have the same symptoms that your child has. It may harm them.
You can ask your pharmacist or healthcare provider for information about LIBERVANT that is written for health professionals.What are the ingredients in LIBERVANT?
Active ingredient: diazepam
Inactive ingredients: benzyl alcohol, clove oil, EDTA disodium salt, FD&C Green #3, glycerol monooleate, hypromellose, peppermint oil, polyethylene oxide, polyvinylpyrrolidone, sodium phosphate, sucralose, vanillin, xanthan gum, water, and white ink
Manufactured by: Aquestive Therapeutics, Warren NJ 07059, USA
For more information, go to www.aquestive.com or call 1-877-394-5045.This Medication Guide is approved by the U.S. Food and Drug Administration Revision Date: 4/2024
-
LIBERVANT is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death. Get emergency help right away if any of the following happens:
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
LIBERVANT™ [lih-ber-vant]
(diazepam) buccal filmFamily members, caregivers, and others who may need to administer LIBERVANT should read this Instructions for Use that comes with LIBERVANT before using it.
This Instructions for Use contains information on how to use LIBERVANT. This information does not take the place of talking to your child’s healthcare provider about your child’s medical condition or your child’s treatment. Ask your child’s healthcare provider or pharmacist if you or others who may need to administer LIBERVANT have any questions about how to use LIBERVANT the right way.
Important Information You Need to Know Before Using LIBERVANT
- For buccal use (place film on inside of cheek)
- Before using LIBERVANT, make sure your child’s healthcare provider shows you the right way to use it. If treatment is unable to be given and there is concern, call for emergency medical help right away.

Preparing to Use LIBERVANT
- Make sure hands are clean and dry before handling LIBERVANT so the film does not stick to your fingers.
- Check expiration date. Do not use if the expiration date has passed.

Using LIBERVANT
Step 1. Open Pouch and Remove Film from Pouch

- Fold foil pouch along solid line. While folded over, note where the vertical slit is and carefully tear down the side of the pouch at the slit along the arrow to open the pouch.
- Remove LIBERVANT film from foil pouch. Each foil pouch contains 1 dose of LIBERVANT.
Step 2. Place 1 Film on Inside of Cheek
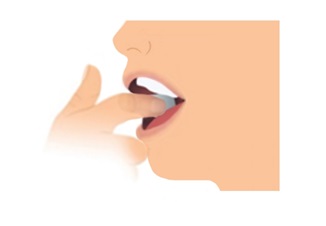
- Do not give LIBERVANT film with liquids.
- Stretch either cheek open with one hand and place one film flat against the cheek with your other hand. Do not rub the film into the cheek with your finger.
- Place the film directly onto the inside of the cheek. Do not place film on teeth.
- Remove fingers from the cheek.
- If the film is spit out or blown out immediately, attempt to give another dose using a new film. If you cannot give the dose, call for emergency help.
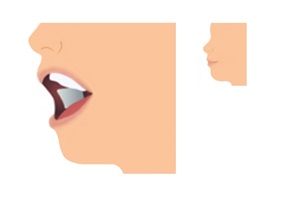
Step 3. Allow Film to Dissolve
- If the film is accidentally swallowed or chewed, there is no need to give a replacement dose.
- LIBERVANT will stick to the inside of the cheek and begin to dissolve.
- The mouth can be closed or remain open while waiting.
- Saliva may be swallowed normally as the film dissolves.
What to do after LIBERVANT has been taken:
- Note the time LIBERVANT was taken and changes in the resting breath rate.
- Your child’s healthcare provider may prescribe a second dose of LIBERVANT. If a second dose is needed, it may be given at least 4 hours after the first dose of LIBERVANT film is given. Repeat Steps 1 through 3.
-
Do not give a second dose if:
◦ you are concerned about your child’s breathing,
◦ an emergency rescue treatment with breathing help is needed,
◦ or there is more sleepiness than normal. -
Call for emergency medical help if any of the following occur:
◦ there is an increase in seizure frequency that does not stop after using LIBERVANT as instructed by your child’s healthcare provider
◦ seizure behavior is different from other episodes
◦ the frequency or severity of the seizure is alarming
◦ either skin color or breathing is alarming
Disposing of LIBERVANT
- Throw away (dispose of) empty foil pouch with the regular trash. Any films that were spit out or not used after opening should be flushed down the toilet or placed in the sink and rinsed with water until film is no longer visible.
For more information about LIBERVANT, go to www.aquestive.com or call 1-877-394-5045.
Manufactured by:
Aquestive Therapeutics
Warren, NJ 07059This Instructions for Use has been approved by the U.S. Food and Drug Administration Issued Date: 4/2024
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 10094-305-01
Libervant
(diazepam) buccal film
5 mg
1 Buccal Film Dose
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 10094-307-01
Libervant
(diazepam) buccal film
7.5 mg
1 Buccal Film Dose
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 10094-310-01
Libervant
(diazepam) buccal film
10 mg
1 Buccal Film Dose
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 10094-312-01
Libervant
(diazepam) buccal film
12.5 mg
1 Buccal Film Dose
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 10094-315-01
Libervant
(diazepam) buccal film
15 mg
1 Buccal Film Dose
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIBERVANT
diazepam filmProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10094-305 Route of Administration BUCCAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIAZEPAM (UNII: Q3JTX2Q7TU) (DIAZEPAM - UNII:Q3JTX2Q7TU) DIAZEPAM 5 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CLOVE OIL (UNII: 578389D6D0) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) HYPROMELLOSES (UNII: 3NXW29V3WO) PEPPERMINT OIL (UNII: AV092KU4JH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM PHOSPHATE (UNII: SE337SVY37) SUCRALOSE (UNII: 96K6UQ3ZD4) VANILLIN (UNII: CHI530446X) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) POVIDONE K90 (UNII: RDH86HJV5Z) EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code D5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10094-305-02 2 in 1 BOX 04/29/2024 1 NDC: 10094-305-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218623 04/29/2024 LIBERVANT
diazepam filmProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10094-307 Route of Administration BUCCAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIAZEPAM (UNII: Q3JTX2Q7TU) (DIAZEPAM - UNII:Q3JTX2Q7TU) DIAZEPAM 7.5 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CLOVE OIL (UNII: 578389D6D0) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) HYPROMELLOSES (UNII: 3NXW29V3WO) PEPPERMINT OIL (UNII: AV092KU4JH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM PHOSPHATE (UNII: SE337SVY37) SUCRALOSE (UNII: 96K6UQ3ZD4) VANILLIN (UNII: CHI530446X) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) POVIDONE K90 (UNII: RDH86HJV5Z) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code D7;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10094-307-02 2 in 1 BOX 04/29/2024 1 NDC: 10094-307-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218623 04/29/2024 LIBERVANT
diazepam filmProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10094-310 Route of Administration BUCCAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIAZEPAM (UNII: Q3JTX2Q7TU) (DIAZEPAM - UNII:Q3JTX2Q7TU) DIAZEPAM 10 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CLOVE OIL (UNII: 578389D6D0) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) HYPROMELLOSES (UNII: 3NXW29V3WO) PEPPERMINT OIL (UNII: AV092KU4JH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM PHOSPHATE (UNII: SE337SVY37) SUCRALOSE (UNII: 96K6UQ3ZD4) VANILLIN (UNII: CHI530446X) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) POVIDONE K90 (UNII: RDH86HJV5Z) EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code D10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10094-310-02 2 in 1 BOX 04/29/2024 1 NDC: 10094-310-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218623 04/29/2024 LIBERVANT
diazepam filmProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10094-312 Route of Administration BUCCAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIAZEPAM (UNII: Q3JTX2Q7TU) (DIAZEPAM - UNII:Q3JTX2Q7TU) DIAZEPAM 12.5 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CLOVE OIL (UNII: 578389D6D0) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) HYPROMELLOSES (UNII: 3NXW29V3WO) PEPPERMINT OIL (UNII: AV092KU4JH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM PHOSPHATE (UNII: SE337SVY37) SUCRALOSE (UNII: 96K6UQ3ZD4) VANILLIN (UNII: CHI530446X) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) POVIDONE K90 (UNII: RDH86HJV5Z) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code D12;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10094-312-02 2 in 1 BOX 04/29/2024 1 NDC: 10094-312-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218623 04/29/2024 LIBERVANT
diazepam filmProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10094-315 Route of Administration BUCCAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIAZEPAM (UNII: Q3JTX2Q7TU) (DIAZEPAM - UNII:Q3JTX2Q7TU) DIAZEPAM 15 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CLOVE OIL (UNII: 578389D6D0) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) HYPROMELLOSES (UNII: 3NXW29V3WO) PEPPERMINT OIL (UNII: AV092KU4JH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM PHOSPHATE (UNII: SE337SVY37) SUCRALOSE (UNII: 96K6UQ3ZD4) VANILLIN (UNII: CHI530446X) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) POVIDONE K90 (UNII: RDH86HJV5Z) EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code D15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10094-315-02 2 in 1 BOX 04/29/2024 1 NDC: 10094-315-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218623 04/29/2024 Labeler - AQUESTIVE THERAPEUTICS (615102303)
Trademark Results [LIBERVANT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIBERVANT 97207918 not registered Live/Pending |
Aquestive Therapeutics, Inc. 2022-01-07 |
 LIBERVANT 90309531 not registered Live/Pending |
Aquestive Therapeutics, Inc. 2020-11-10 |
 LIBERVANT 87492329 not registered Live/Pending |
AQUESTIVE THERAPEUTICS, INC. 2017-06-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.