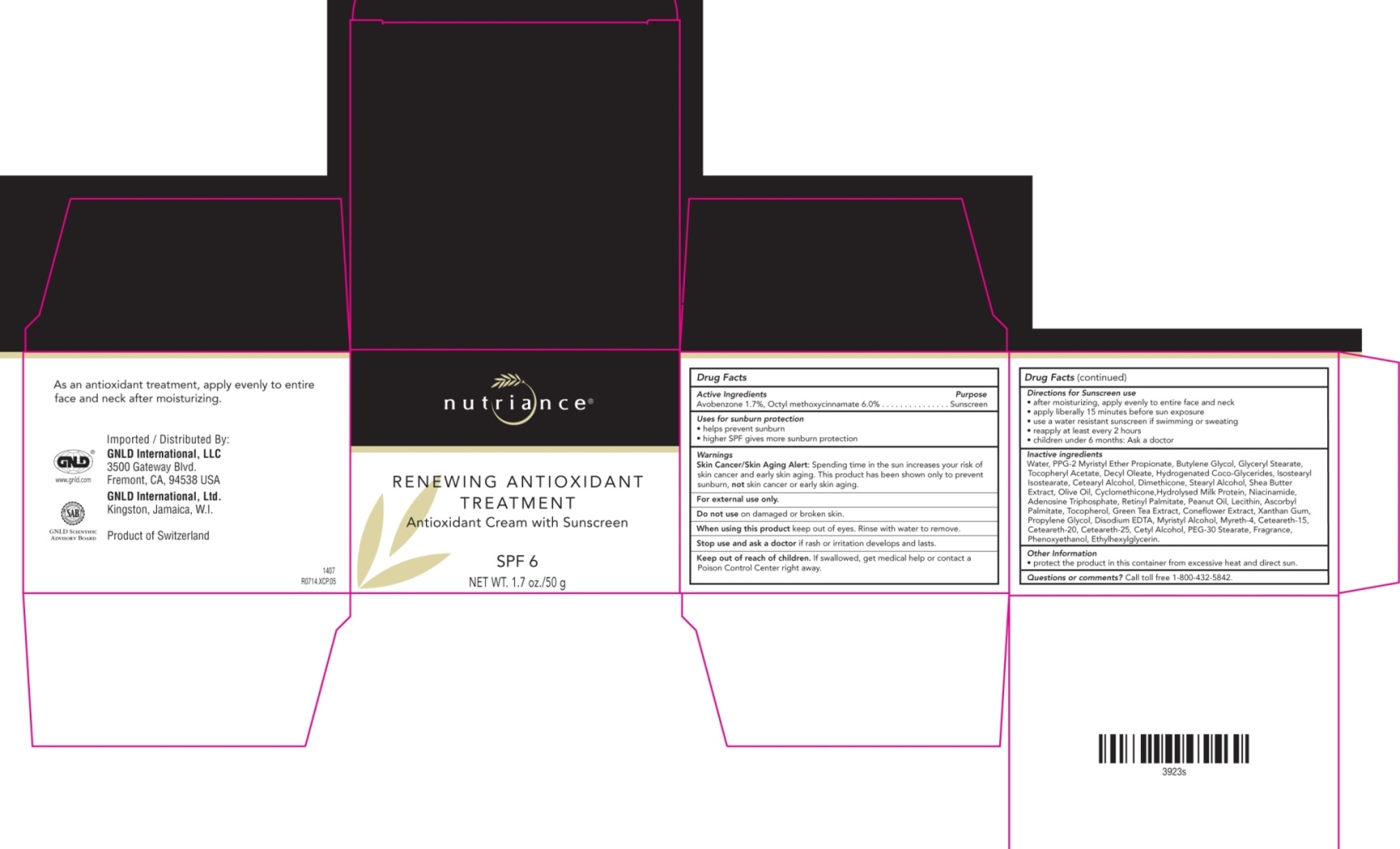
RENEWING ANTIOXIDANT TREATMENT SPF 6 GNLD INTERNATIONAL- avobenzone, octyl methoxycinnamate cream
Renewing Antioxidant Treatment SPF 6 by
Drug Labeling and Warnings
Renewing Antioxidant Treatment SPF 6
by is a Otc medication manufactured, distributed, or labeled by Temmentec AG,
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active Ingredients Purpose
Avobenzone 1.7%, Octyl methoxycinnamate 6.0% . . . . . . . . . . . . . . . Sunscreen
Uses for sunburn protection
helps prevent sunburn
higher SPF gives more sunburn protection
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if rash or irritation develops and lasts
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown to only prevent sunburn, not skin cancer or early skin aging.
For External use only
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove
Directions for Sunscreen use
after moisturizing, apply evenly to entire face and neck
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months: Ask a doctor
Inactive ingredients
Water, PPG-2 Myristyl Ether Propionate, Butylene Glycol, Glyceryl Stearate, Tocopheryl Acetate, Decyl Oleate, Hydrogenated Coco-Glycerides, Isostearyl Isostearate, Cetearyl Alcohol, Dimethicone, Stearyl Alcohol, Shea Butter Extract, Olive Oil, Cyclomethicone,Hydrolysed Milk Protein, Niacinamide, Adenosine Triphosphate, Retinyl Palmitate, Peanut Oil, Lecithin, Ascorbyl Palmitate, Tocopherol, Green Tea Extract, Coneflower Extract, Xanthan Gum, Propylene Glycol, Disodium EDTA, Myristyl Alcohol, Myreth-4, Ceteareth-15, Ceteareth-20, Ceteareth-25, Cetyl Alcohol, PEG-30 Stearate, Fragrance, Phenoxyethanol, Ethylhexylglycerin.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RENEWING ANTIOXIDANT TREATMENT SPF 6 GNLD INTERNATIONAL
avobenzone, octyl methoxycinnamate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68807-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.7 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DECYL OLEATE (UNII: ZGR06DO97T) HYDROGENATED COCO-GLYCERIDES (UNII: XDD37N2GPR) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SHEANUT OIL (UNII: O88E196QRF) OLIVE OIL (UNII: 6UYK2W1W1E) CYCLOMETHICONE (UNII: NMQ347994Z) NIACINAMIDE (UNII: 25X51I8RD4) ADENOSINE TRIPHOSPHATE (UNII: 8L70Q75FXE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PEANUT OIL (UNII: 5TL50QU0W4) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRETH-4 (UNII: BJT811863E) CETEARETH-15 (UNII: 867H4YOZ8Z) CETEARETH-25 (UNII: 8FA93U5T67) CETYL ALCOHOL (UNII: 936JST6JCN) PEG-30 STEARATE (UNII: 1U8KB35S20) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68807-102-02 1 in 1 CARTON 1 NDC: 68807-102-01 50 g in 1 JAR; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2014 Labeler - Temmentec AG (480586411) Registrant - <text xmlns="urn:hl7-org:v3"> <paragraph/> <paragraph>Active Ingredients Purpose</paragraph> <paragraph>Avobenzone 1.7%, Octyl methoxycinnamate 6.0% . . . . . . . . . . . . . . . Sunscreen</paragraph></text> (948995019) Establishment Name Address ID/FEI Business Operations Temmentec Ag 480586411 manufacture(68807-102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.