ZOVIRAX- acyclovir cream
Zovirax by
Drug Labeling and Warnings
Zovirax by is a Prescription medication manufactured, distributed, or labeled by Bausch Health US, LLC, Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZOVIRAX Cream safely and effectively. See full prescribing information for ZOVIRAX Cream.
ZOVIRAX® (acyclovir) cream 5% for topical use
Initial U.S. Approval: 2002INDICATIONS AND USAGE
ZOVIRAX Cream is a herpes simplex virus (HSV) nucleoside analogue DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in immunocompetent adults and adolescents 12 years of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Topical cream containing 5% acyclovir. (3)
CONTRAINDICATIONS
- ZOVIRAX Cream is contraindicated in patients with known hypersensitivity to acyclovir, valacyclovir or any component of the formulation. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- The most common adverse reactions reported were local skin reactions at the application site. (6.1)
- Angioedema, anaphylaxis, contact dermatitis and eczema have been reported. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Clinical experience has identified no interactions resulting from topical or systemic administration of other drugs concomitantly with ZOVIRAX Cream. Due to minimal systemic absorption of ZOVIRAX Cream, systemic drug interactions are unlikely. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 General
5.2 Contact Sensitization
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Subjects
14.2 Pediatric Subjects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 General
ZOVIRAX Cream should only be applied on the affected external aspects of the lips and face in patients with herpes labialis. Because no data are available, application to human mucous membranes is not recommended. ZOVIRAX Cream is intended for cutaneous use only and should not be used in the eye or inside the mouth or nose.
5.2 Contact Sensitization
ZOVIRAX Cream has a potential for irritation and contact sensitization [see Adverse Reactions (6.1)].
The effect of ZOVIRAX Cream has not been established in immunocompromised patients.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
In five double-blind, placebo-controlled trials, 1124 patients were treated with ZOVIRAX Cream and 1161 with placebo (vehicle) cream. Local application site reactions were reported by 5% of patients receiving ZOVIRAX Cream and 4% of patients receiving placebo. The most common adverse reactions at the site of topical application were dry lips, desquamation, dryness of skin, cracked lips, burning skin, pruritus, flakiness of skin, and stinging on skin; each adverse reaction occurred in less than 1% of patients receiving ZOVIRAX Cream and placebo. Three patients on ZOVIRAX Cream and one patient on placebo discontinued treatment due to an adverse event.
An additional study, enrolling 22 healthy adults, was conducted to evaluate the dermal tolerance of ZOVIRAX Cream compared with vehicle using single occluded and semi-occluded patch testing methodology. Both ZOVIRAX Cream and placebo showed a high and cumulative irritation potential. Another study, enrolling 251 healthy adults, was conducted to evaluate the contact sensitization potential of ZOVIRAX Cream using repeat insult patch testing methodology. Of 202 evaluable subjects, possible cutaneous sensitization reactions were observed in the same 4 (2%) subjects with both ZOVIRAX Cream and placebo, and these reactions to both ZOVIRAX Cream and placebo were confirmed in 3 subjects upon rechallenge. The sensitizing ingredient(s) has not been identified.
The safety profile in patients 12 to 17 years of age was similar to that observed in adults.
6.2 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of acyclovir cream. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to acyclovir cream.
General: Angioedema, anaphylaxis.
Skin: Contact dermatitis, eczema.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
It is not known whether topically applied acyclovir is excreted in breast milk. Systemic exposure following topical administration is minimal.
However, after oral administration of ZOVIRAX, acyclovir concentrations have been documented in breast milk in two women and ranged from 0.6 to 4.1 times the corresponding plasma levels. These concentrations would potentially expose the nursing infant to a dose of acyclovir up to 0.3 mg/kg/day. Nursing mothers who have active herpetic lesions near or on the breast should avoid nursing.
8.4 Pediatric Use
An open-label, uncontrolled trial with ZOVIRAX Cream was conducted in 113 patients aged 12 to 17 years with recurrent herpes labialis. In this trial, therapy was applied using the same dosing regimen as in adults and subjects were followed for adverse events. The safety profile was similar to that observed in adults. Safety and effectiveness in pediatric patients less than 12 years of age have not been established.
8.5 Geriatric Use
Clinical studies of acyclovir cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Systemic absorption of acyclovir after topical administration is minimal [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Overdosage by topical application of ZOVIRAX Cream is unlikely because of minimal systemic exposure [see Clinical Pharmacology (12.3)]. There is no information available for overdose.
-
11 DESCRIPTION
ZOVIRAX is the brand name for acyclovir, a synthetic nucleoside analogue active against herpes viruses. ZOVIRAX Cream 5% is a formulation for topical administration.
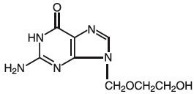
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy) methyl]-6H-purin-6-one; it has the following structural formula:
Acyclovir is a white, crystalline powder with the molecular formula C8H11N5O3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5 mg/mL. The pKa's of acyclovir are 2.27 and 9.25.
Each gram of ZOVIRAX Cream 5% contains 50 mg of acyclovir and the following inactive ingredients: cetostearyl alcohol, mineral oil, poloxamer 407, propylene glycol, sodium lauryl sulfate, water and white petrolatum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Acyclovir is an antiviral drug active against herpes simplex virus [see Microbiology (12.4)].
12.3 Pharmacokinetics
A clinical pharmacology study was performed with ZOVIRAX Cream in adult volunteers to evaluate the percutaneous absorption of acyclovir. In this study, which included 6 male volunteers, the cream was applied to an area of 710 cm2 on the backs of the volunteers 5 times daily at intervals of 2 hours for a total of 4 days. The weight of cream applied and urinary excretion of acyclovir were measured daily. Plasma concentration of acyclovir was assayed 1 hour after the final application. The average daily urinary excretion of acyclovir was approximately 0.04% of the daily applied dose. Plasma acyclovir concentrations were below the limit of detection (0.01 μM) in 5 subjects and barely detectable (0.014 μM) in 1 subject. Systemic absorption of acyclovir from ZOVIRAX Cream is minimal in adults.
The systemic absorption of acyclovir following topical application of cream has not been evaluated in patients <18 years of age.
12.4 Microbiology
Mechanism of Action: Acyclovir is a synthetic purine nucleoside analogue with cell culture and in vivo inhibitory activity against HSV types 1 (HSV-1) and 2 (HSV-2).
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In cell culture, acyclovir triphosphate stops replication of herpes viral DNA. This inhibition is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase.
Antiviral Activity: The quantitative relationship between the cell culture susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the EC50 values against herpes simplex virus isolates range from 0.09 to 59.9 μM (0.02 to 13.5 μg/mL) for HSV-1 and from 0.04 to 44.0 μM (0.01 to 9.9 μg/mL) for HSV-2.
Drug Resistance: Resistance of HSV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir‑resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Systemic exposure following topical administration of acyclovir is minimal. Dermal carcinogenicity studies were not conducted. Results from the studies of carcinogenesis, mutagenesis and fertility are not included in the full prescribing information for ZOVIRAX Cream due to the minimal exposures of acyclovir that result from dermal application. Information on these studies is available in the full prescribing information for ZOVIRAX Capsules, Tablets, and Suspension and ZOVIRAX for Injection.
-
14 CLINICAL STUDIES
14.1 Adult Subjects
ZOVIRAX Cream was evaluated in two double-blind, randomized, placebo (vehicle)-controlled trials for the treatment of recurrent herpes labialis. The average patient had five episodes of herpes labialis in the previous 12 months. In the first trial, the median age of subjects was 37 years (range 18 to 81 years), 74% were female, and 94% were Caucasian. In the second trial, median age of subjects was 38 years (range 18 to 87 years), 73% were female, and 94% were Caucasian. Subjects were instructed to initiate treatment within 1 hour of noticing signs or symptoms and continue treatment for 4 days, with application of study medication 5 times per day. In both studies, the mean duration of the recurrent herpes labialis episode was approximately one-half day shorter in the subjects treated with ZOVIRAX Cream (n = 682) compared with subjects treated with placebo (n = 703) for approximately 4.5 days versus 5 days, respectively. No significant difference was observed between subjects receiving ZOVIRAX Cream or placebo in the prevention of progression of cold sore lesions.
14.2 Pediatric Subjects
An open-label, uncontrolled trial with ZOVIRAX Cream was conducted in 113 patients aged 12 to 17 years with recurrent herpes labialis. In this trial, therapy was applied using the same dosing regimen as in adults and subjects were followed for adverse events. The safety profile was similar to that observed in adults.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each gram of ZOVIRAX Cream 5% contains 50 mg acyclovir in an aqueous cream base. ZOVIRAX Cream is supplied as follows:
- 5 g tubes NDC: 0187-0994-45
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
General
Patients should be informed that ZOVIRAX Cream is a prescription topical cream for the treatment of cold sores (recurrent herpes labialis) that occur on the face and lips. ZOVIRAX Cream is not a cure for cold sores. Patients should be instructed that ZOVIRAX Cream is intended for cutaneous use only for herpes labialis of the lips and around the mouth. Patients should be advised that ZOVIRAX Cream should not be used in the eye, inside the mouth or nose, or on the genitals. Patients should be instructed to avoid applying other topical products to the affected area while using ZOVIRAX Cream.
Do not use if you are allergic to ZOVIRAX Cream or any of the ingredients in ZOVIRAX Cream. Before you use ZOVIRAX Cream, tell your doctor if you are pregnant, planning to become pregnant, or are breast-feeding.
Instructions for Use
Treatment should be initiated at the earliest sign or symptom of recurrence. Patients should be instructed to wash hands prior to application and ensure the face and/or lips are clean and dry. Patients should be advised to apply ZOVIRAX Cream topically 5 times per day for 4 days. Patients should be instructed to topically apply a quantity of ZOVIRAX Cream sufficient to cover the affected area, including the outer margin. Patients should be advised to avoid unnecessary rubbing of the affected area to avoid aggravating or transferring the infection. Patients should be instructed to wash their hands with soap and water after using ZOVIRAX Cream. Keep out of reach of children.
Possible Side Effects
Common skin-related side effects that occurred when ZOVIRAX Cream was applied include application site reactions. ZOVIRAX Cream has the potential for irritation and contact sensitization.
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATIONZOVIRAX (zho-vahy-rex)(acyclovir) Cream 5%
Important information: ZOVIRAX Cream is for use on cold sores on the lips and around the mouth only. ZOVIRAX Cream should not be used in your eyes, mouth, nose, or on your genitals.
What is ZOVIRAX Cream?
- ZOVIRAX Cream is a prescription medicine used to treat cold sores (herpes labialis) that are recurring in adults and children 12 years of age and older, and who have normal immune systems.
- ZOVIRAX Cream is not a cure for cold sores.
- It is not known if ZOVIRAX Cream is safe and effective in children less than 12 years of age.
Who should not use ZOVIRAX Cream?
Do not use ZOVIRAX Cream if you are:
- allergic to ZOVIRAX Cream or any of the ingredients in ZOVIRAX Cream. See the end of this leaflet for a complete list of ingredients in ZOVIRAX Cream.
What should I tell my healthcare provider before using ZOVIRAX Cream?
Before using ZOVIRAX Cream, tell your healthcare provider about all of your medical conditions, including if you:
- become sick very easily (have a weak immune system).
- are pregnant or plan to become pregnant. It is not known if ZOVIRAX Cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ZOVIRAX Cream passes into your breast milk. You should not breastfeed if you have a cold sore near or on your breast.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ZOVIRAX Cream?
- Use ZOVIRAX Cream exactly as your healthcare provider tells you to use it.
- Use ZOVIRAX Cream as soon as you have the first symptoms of a cold sore such as itching, redness, burning or tingling, or when the cold sore appears.
- Wash your hands with soap and water before and after applying ZOVIRAX Cream.
- The affected area should be clean and dry before applying ZOVIRAX Cream.
- Apply ZOVIRAX Cream to the affected area 5 times each day for 4 days, including the outer edge.
- You should not apply other skin products to the affected area during treatment with ZOVIRAX Cream.
- Do not rub the cold sore because this may cause the cold sore to spread to other areas around your mouth or make your cold sore worse.
What are the possible side effects of ZOVIRAX Cream?
The most common side effects of ZOVIRAX Cream are skin reactions at the treatment site and may include: dry or cracked lips, peeling, flaking or dryness of the skin, a burning or stinging feeling, and itching.
These are not all the possible side effects of ZOVIRAX Cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZOVIRAX Cream?
- Store ZOVIRAX Cream at room temperature between 68° to 77°F (20° to 25°C).
- Keep ZOVIRAX Cream and all medicines out of reach of children.
General information about the safe and effective use of ZOVIRAX Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZOVIRAX Cream for a condition for which it was not prescribed. Do not give ZOVIRAX Cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZOVIRAX Cream that is written for health professionals.
What are the ingredients in ZOVIRAX Cream?
Active ingredient: acyclovir
Inactive ingredients: cetostearyl alcohol, mineral oil, poloxamer 407, propylene glycol, sodium lauryl sulfate, water, and white petrolatum
Manufactured for: Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
By: Valeant Pharmaceuticals International, Inc.
Laval, Quebec H7L 4A8, Canada
Zovirax is a trademark of GlaxoSmithKline LLC used under license.
© Valeant Pharmaceuticals North America LLC
For more information, call 1-800-321-4576.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2018
9462202 - PRINCIPAL DISPLAY PANEL - 5g Tube Carton
-
INGREDIENTS AND APPEARANCE
ZOVIRAX
acyclovir creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0187-0994 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength acyclovir (UNII: X4HES1O11F) (acyclovir - UNII:X4HES1O11F) acyclovir 50 mg in 1 g Inactive Ingredients Ingredient Name Strength Cetostearyl alcohol (UNII: 2DMT128M1S) Mineral oil (UNII: T5L8T28FGP) Poloxamer 407 (UNII: TUF2IVW3M2) Sodium lauryl sulfate (UNII: 368GB5141J) Water (UNII: 059QF0KO0R) Petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-0994-45 1 in 1 CARTON 12/30/2002 1 5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0187-0994-20 1 in 1 CARTON 12/30/2002 05/31/2015 2 0.9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021478 12/30/2002 Labeler - Bausch Health Americas, Inc. (042230623) Establishment Name Address ID/FEI Business Operations GlaxoSmithKline Inc. 205556368 MANUFACTURE(0187-0994) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 245141858 MANUFACTURE(0187-0994)
Trademark Results [Zovirax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZOVIRAX 98588614 not registered Live/Pending |
Rising Pharma Holdings, Inc. 2024-06-06 |
 ZOVIRAX 85161519 not registered Dead/Abandoned |
GlaxoSmithKline LLC 2010-10-26 |
 ZOVIRAX 75282515 2223200 Dead/Cancelled |
Glaxo Wellcome Inc. 1997-04-28 |
 ZOVIRAX 73203076 1141502 Live/Registered |
Burroughs Wellcome Co. 1979-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.