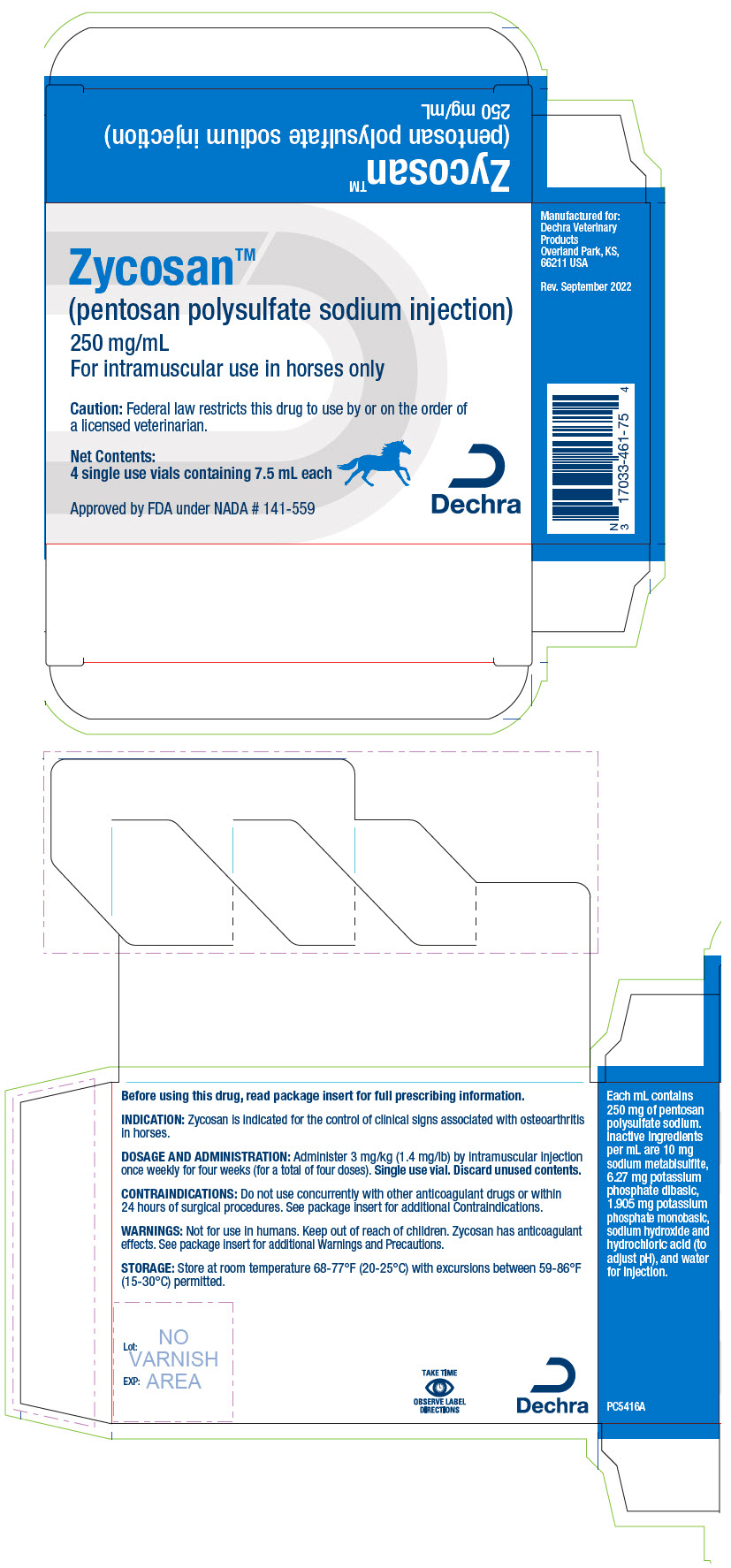
ZYCOSAN- pentosan polysulfate sodium injection, solution
Zycosan by
Drug Labeling and Warnings
Zycosan by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
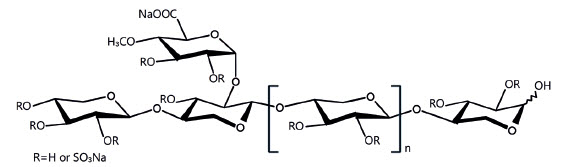
Zycosan contains pentosan polysulfate sodium, a semi-synthetic polysulfated xylan. It is a pale yellow to brownish yellow, clear, sterile solution. Each milliliter of Zycosan contains 250 mg of pentosan polysulfate sodium. Inactive ingredients per mL are 10 mg sodium metabisulfite, 6.27 mg potassium phosphate dibasic, 1.905 mg potassium phosphate monobasic, sodium hydroxide and hydrochloric acid (to adjust pH), and water for injection. The molecular weight of pentosan polysulfate sodium is 4000 - 7500 Daltons.
The structural formula is:
- INDICATION:
- DOSAGE AND ADMINISTRATION:
-
CONTRAINDICATIONS:
Horses with hypersensitivity to pentosan polysulfate sodium or any of the inactive ingredients in Zycosan should not receive Zycosan. Do not use Zycosan concurrently with other anticoagulant drugs. Do not use in horses with clotting disorders or within 24 hours of surgical procedures (see Warnings and Precautions).
-
WARNINGS AND PRECAUTIONS:
User Safety Warnings:
Not for use in humans. Keep out of reach of children. Pentosan polysulfate sodium is a weak anticoagulant. Caution should be used when administering Zycosan if you are taking an anticoagulant. In case of accidental self-injection, seek immediate medical attention. If product comes into contact with skin, rinse skin thoroughly with water and seek medical attention if needed. To obtain a Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
Animal Safety Warnings and Precautions:
Zycosan has been shown to prolong coagulation parameters up to 24 hours after injection, therefore caution should be used when administering this drug before or after strenuous activities (see Target Animal Safety). Due to the anticoagulant effects, this drug may exacerbate Exercise Induced Pulmonary Hemorrhage (EIPH).
The concurrent use of NSAIDs with Zycosan has not been evaluated. Due to the anticoagulant effects of Zycosan and known anticoagulant effects of some NSAIDs, caution should be used if NSAIDs are concurrently administered. Horses concurrently treated with Zycosan and NSAIDs should be monitored for hemorrhage or other clinical signs of abnormal bleeding (e.g., petechiae, ecchymosis, or epistaxis).
The safety of long-term repeat use of Zycosan has not been evaluated.
Pigmentary changes in the retina (pigmentary maculopathy) have been reported in human patients following long-term oral use of pentosan polysulfate sodium. It is not known if a similar finding occurs in horses.
The safe use of Zycosan has not been evaluated in breeding, pregnant, or lactating horses.
-
ADVERSE REACTIONS:
In a clinical field effectiveness study, two hundred thirty-seven horses (120 Zycosan and 117 saline control) were evaluated for field safety (see Effectiveness). All doses of Zycosan were administered in the neck muscle.
Injection site reactions were the most frequently reported adverse reactions during the study. Injection site reactions were associated with clinicopathology changes in some cases. Other adverse reactions reported in more than one horse were prolongation of coagulation parameters (activated partial thromboplastin time (aPTT) and prothrombin time (PT)), lethargy, behavior changes, and colic. Adverse reactions are summarized in Table 1. Horses may have experienced more than one of the observed adverse reactions.
Table 1: Adverse Reactions Adverse Reaction Number (%) of Zycosan™ treated horses (N=120) Number (%) of Saline treated horses (N=117) - * Occurring 0-3 hours post-injection; clinical signs included pain, heat, swelling, edema, redness, or neck muscle cramping. Horses may have experienced more than one episode.
- † Occurring more than 3 hours post-injection; observations included pain, heat, swelling, edema, redness, or neck muscle cramping. Pain was exhibited local to the injection site and as reluctance to eat, drink, or move the neck or head. Horses may have experienced more than one episode.
- ‡ Observations included aggression, stomping, pawing, agitation, anxiousness, overactivity, quietness and/or depression, or unsettledness.
Immediate or Peri-Dosing Injection Site Reaction* 21 (18%) 4 (3%) Delayed Injection Site Reaction† 13 (11%) 3 (3%) Prolonged aPTT
(post-treatment)18 (15%) 1 (1%) Prolonged PT
(post-treatment)5 (4%) 1 (1%) Lethargy 14 (12%) 7 (6%) Behavior Change ‡ 10 (8%) 8 (7%) Colic 2 (2%) 0 (0%) Elevated Sorbitol Dehydrogenase (SDH) 1 (1%) 0 (0%) Stiffness 1 (1%) 0 (0%) Injection site reactions (heat, pain, swelling/edema, or redness) occurred more frequently and were generally more severe in Zycosan treated horses as compared to control horses over the course of the study. Several Zycosan treated horses had injection site reactions following more than one injection. The onset of reactions ranged from 0 hours to 3 days post injection. The duration of the reactions ranged from 1 to 5 days. Most reactions resolved without treatment.
Injection site reactions in Zycosan treated horses were predominantly characterized by swelling/edema ranging in size from 0.2 cm to 15 cm at their widest point. Pain was the most commonly observed concurrent clinical sign associated with the swelling/edema in Zycosan treated horses. Pain was generally exhibited local to the injection site and as reluctance to eat, drink, or move the neck or head. Lethargy or depression were reported concurrently in some horses. One Zycosan treated horse had neck muscle cramping observed concurrently.
One Zycosan treated horse experienced swelling accompanied by heat and pain at the injection site along with mild hyperglycemia, an increase in its white blood cell count and neutrophilia. This horse recovered without treatment.
Two Zycosan treated horses developed large (15 cm) swellings along with pain and heat at the injection site beginning 1 to 2 days following injection. Both horses concurrently showed mild hyperbilirubinemia, mild hyperglycemia, mild neutrophilia, and mild monocytosis on clinical pathology. One of these horses was reluctant to move its head or neck and was noted to be tachypneic the day following injection. The second horse showed concurrent clinical signs of anorexia, depression, and fever. Both horses were removed from the study and treated with flunixin meglumine. The horse with concurrent fever was also treated with an oral antibiotic for 5 days. Both horses recovered within 5 days from the onset of clinical signs.
Coagulation parameters were evaluated pre-treatment and 3 hours post-treatment following the first (study day 0), third (study day 14) and fourth (study day 21) injection. Mean post-treatment values for aPTT in the Zycosan treated group increased by approximately 19 seconds at each study timepoint but remained within the laboratory reference range.
Clinically relevant prolongation of aPTT values occurred post-treatment in 18 Zycosan treated horses, with some horses experiencing prolongation of aPTT at multiple timepoints. Clinically relevant prolongation in PT occurred post-treatment in 5 Zycosan treated horses. Three Zycosan treated horses showed concurrent clinically relevant prolongation in aPTT and PT at study day 0 (N=1) or study day 14 (N=2). One of the horses with post-treatment prolongation of aPTT and PT at study day 14 was concurrently reported to have an injection site reaction. Clinical signs of bleeding or thrombocytopenia were not observed in any horses with prolongation of coagulation parameters.
Two Zycosan treated horses developed clinical signs of colic (lethargy, generalized discomfort, decreased appetite, decreased water intake, and/or decreased manure output) within 12 hours following treatment after the third injection. One horse was diagnosed with a pelvic flexure impaction. One horse was noted to display a markedly lowered head position prior to colic signs and was removed from the study. In both cases, colic signs resolved within 24 hours with symptomatic treatment.
One Zycosan treated horse showed an increase in SDH in conjunction with trending increases in aspartate aminotransferase (AST) and alanine transaminase (ALT) that did not exceed the reference range. Concurrent clinically relevant changes in γ-glutamyl transferase (GGT) or clinical signs were not observed in this horse.
-
CONTACT INFORMATION:
Contact Dechra at (866) 933-2472 or www.dechra-us.com for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS). To report suspected adverse drug experiences, contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY:
Pentosan polysulfate sodium is a low molecular weight heparin-like compound. It is chemically and structurally similar to heparin and other glycosaminoglycans (GAG). Pentosan polysulfate sodium has anticoagulant and fibrinolytic effects. The mechanism of action for pentosan polysulfate sodium is unknown but is thought to include stimulation of hyaluronic acid and GAG synthesis in damaged joints, inhibition of proteolytic enzymes (including metalloproteinases), and scavenging of free radicals. Pentosan polysulfate sodium may also modulate receptor-mediated binding of cytokines.
-
EFFECTIVENESS:
Two hundred and thirty-seven (237) client-owned horses with osteoarthritis (82 non-pregnant/non-lactating mares, 151 geldings, and 4 stallions), aged 3-32 years old, of various breeds, and weighing between 153-904 kg (337 to 1989 pounds) were enrolled in a controlled, randomized, masked, multi-site field study. One hundred and twenty (120) horses received Zycosan administered at 3 mg/kg (1.4 mg/lb) via intramuscular injection and 117 horses received a volume matched negative (saline) control. All intramuscular injections were administered in the neck once weekly for four weeks. Two hundred and twenty-two (222) horses (109 Zycosan and 113 saline control) were included in the evaluation of effectiveness (final effectiveness analysis).
Enrolled horses had a unilateral lameness between Grade 2 and 4 (≥2 and ≤4) on the American Association of Equine Practitioners (AAEP) Lameness Scale1 and a diagnosis of osteoarthritis based on a lameness examination and radiographs. Nerve blocks were permitted to confirm and localize the clinical lameness.
Horses with prior diagnosis of bleeding issues (including exercise induced pulmonary hemorrhage (EIPH)), or those where trauma or bleeding were expected to occur during the study time-period (e.g., from planned surgery) were not enrolled. Horses receiving systemic non-steroidal anti-inflammatory drugs at the start of the study were not enrolled.
Horses were assigned a baseline AAEP lameness grade at enrollment (study day 0) and were evaluated for lameness at study days 7, 14, 21, and 28. Horses were considered a treatment success if the baseline lameness grade in the identified limb improved by ≥ 1 AAEP lameness grade on Day 28.
Table 2 summarizes the treatment success rate in each treatment group. The treatment success rate was 57% for horses in the Zycosan group and 36% in the saline control group.
Table 2: Day 28 Treatment Success Rates Treatment Group Number of Horses Percent Success Zycosan 109 57.00% Negative Control
(saline)113 36.26% The difference in the success rates between the two treatments was not statistically significant (p=0.0548). However, the point estimates of the treatment success rate appeared to indicate a clinically relevant effect size. Sensitivity analyses showed that due to variability across sites, the results varied depending on the inclusion of a small number of cases (n=3). The persuasive size of the effect in the larger proportion of the study supports the conclusion that this field study demonstrated substantial evidence of effectiveness.
- 1 AAEP lameness grades are defined as follows: 0: Lameness not perceptible under any circumstances; 1: Lameness is difficult to observe and is not consistently apparent, regardless of circumstances (e.g., under saddle, circling, inclines, hard surface, etc.); 2: Lameness is difficult to observe at a walk or when trotting in a straight line but consistently apparent under certain circumstances (e.g. weight-carrying, circling, inclines, hard surface, etc.); 3: Lameness is consistently observable at a trot under all circumstances; 4: Lameness is obvious at a walk; 5: Lameness produces minimal weight bearing in motion and/or at rest or a complete inability to move.
-
TARGET ANIMAL SAFETY:
In a laboratory margin of safety study, Zycosan or saline control was administered to 32 healthy adult horses aged 2 to 7 years, in the neck muscle at 3 mg/kg (1× maximum exposure dose, 8 horses), 9 mg/kg (3×, 8 horses), and 15 mg/kg (5×, 8 horses) once weekly for 12 weeks. Eight horses in a control group were administered saline at a volume equivalent to the dosing of the 5× horses.
Pain and swelling at the injection site were noted in all Zycosan treated horses at various study timepoints. At study day 21 (after 4 doses), all 5× horses (8/8) had injection site reactions and 5/8 of the 1× horses and 5/8 of the 3× horses had injection site reactions consisting of pain and swelling.
In three horses receiving a 5× dose, the pain on injection was associated with muscle spasms and stiffness, holding head low, lethargy, and decreased feed intake. The average injection site reaction lasted between 2-6 days in the 5× group; 0-4 days in the 3× group; and 0-2 days in the 1× group. One 3× horse required treatment with flunixin meglumine and oral electrolytes due to pain and swelling at the injection site.
Treatment related effects included a dose dependent trend of prolonged activated partial thromboplastin time (aPTT). Prothrombin time (PT) showed mild increases in the 5× group. During the study, coagulation parameters were measured at 6 and 24 hours post-administration of Zycosan on study days 0, 28, and 56. At 6-hours post administration of Zycosan on study day 28 and 56, aPTT values in the horses administered 15 mg/kg Zycosan (5×) were 190 seconds (laboratory reference range 28-44 seconds). By 24 hours post-injection, aPTT values in the 5× horses remained high, with values ranging from 57.1 to 76.9 seconds. Horses in the 3× group had at least a 2-fold prolongation compared with pre-dose values, with values ranging from 60.9 to 190 seconds at 6-hours post-administration on study day 28, and with values ranging from 67.3 to 165.4 seconds at 6 hours post-administration on study day 56. Minimally prolonged aPTT, as compared with pre-dose values, was noted in all horses in the 1× dose group at 6 hours post-dose timepoint on study days 0, 28, and 56 (average values ranged from 38.9 to 48.9 seconds). aPTT values for the 1× group returned to the normal reference range at 24 hours post-injection. No horses exhibited clinical signs of coagulopathy.
SDH and GGT values in the 5× dose group were higher when compared to the control group. Increased GGT values for the 5× horses stayed within the laboratory reference range. Three study horses (two 5× and one 3×) had SDH values increased above the reference range during the study.
- STORAGE CONDITIONS:
-
HOW SUPPLIED:
Zycosan (pentosan polysulfate sodium injection) is supplied in cartons with each carton containing four clear glass vials with 7.5 mL (1,875 mg) of pentosan polysulfate sodium per vial.
NDC: 17033-461-75
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 7.5 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
ZYCOSAN
pentosan polysulfate sodium injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-461 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENTOSAN POLYSULFATE SODIUM (UNII: 914032762Y) (Pentosan polysulfate - UNII:F59P8B75R4) PENTOSAN POLYSULFATE SODIUM 250 mg in 1 mL Product Characteristics Color YELLOW (clear and pale yellow to brownish yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-461-75 4 in 1 CARTON 1 7.5 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141559 05/01/2023 Labeler - Dechra Veterinary Products (362142734)
Trademark Results [Zycosan]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZYCOSAN 79321757 not registered Live/Pending |
Dechra Limited 2021-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.