EAZI-BREED CIDR SHEEP INSERT- progesterone insert, extended release
EAZI-BREED CIDR Sheep by
Drug Labeling and Warnings
EAZI-BREED CIDR Sheep by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Drug information
EAZI-BREED™
CIDR®
(progesterone) Sheep Insert
NET CONTENTS: 20 EAZI-BREED CIDR Sheep Inserts per bag
Each EAZI-BREED CIDR Sheep Insert contains 0.3 gram of
progesterone in molded silicone over a flexible nylon spine.
Attached to each EAZI-BREED CIDR Sheep Insert is a nylon tail.
NADA 141-302, Approved by FDA
- Active Ingredient
-
Use
Induction of estrus in ewes (sheep) during seasonal anestrus. Seasonal anestrus is when ewes do not have regular estrous cycles outside the natural breeding season. EAZI-BREED CIDR Sheep Inserts have not been tested in estrous cycling ewes.
Read booklet label before using drug for complete product information.
-
WARNINGS
Human Warning: Avoid contact with skin by wearing protective gloves when handling the inserts.
Not for Use in Humans.
Keep this and all medications out of the reach of children.
Environmental Warning: Used (removed) EAZI-BREED CIDR Sheep Inserts still contain some progesterone. Used EAZI-BREED CIDR Sheep Inserts must be stored in a sealable container until disposed. Sealed bag/container with used EAZI-BREED CIDR Sheep Inserts must be properly disposed in accordance with applicable local, state and Federal regulations.
Residue Warning: A pre-slaughter withdrawal period is not required when this product is used according to label directions.
-
Other Information
Store at controlled room temperature
20° to 25° C (68° to 77° F) with excursions
between 15° to 30° C (59° to 86° F). To report
suspected adverse reactions or questions call Zoetis at 1-888-963-8471.
Inactive Ingredients: silicone rubber, nylon.
Made in New Zealand Dist. by: Zoetis Inc.
Kalamazoo, MI 49007
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
-
Warnings
DO NOT USE
in ewes with abnormal, immature or infected genital tracts
in ewes that have never lambed
an insert more than once. To prevent the potential transmission of venereal and blood borne diseases the EAZI-BREED CIDR Sheep
Insert should be disposed after a single use.
WHEN USING THIS PRODUCT
In ewes that respond to treatment the onset of estrus generally occurs within 1 to 3 days after removal of the EAZI-BREED CIDR
Sheep Insert.
Make sure to have a sufficient number of rams to adequately breed all ewes with an induced estrus. Breeds of rams may vary in libido
in the non-breeding season. Therefore a ewe to ram ratio up to 18:1 is recommended for multi-sire situations. For single sire lots, 12:1
for ram lambs and up to 18:1 for yearling rams are recommended.
YOU MAY NOTICE:
Clear, cloudy, yellow or bloody mucus on the outside of EAZI-BREED CIDR Sheep Insert when removed from ewes. The mucus may have an offensive odor. This is a result of mild irritation to the vaginal lining by the presence of the EAZI-BREED CIDR Sheep Insert, and generally clears between the time of removal and breeding. Such irritation does not affect fertility.
-
DIRECTIONS
For induction of estrus in ewes (sheep) during seasonal anestrus:
Administer one EAZI-BREED CIDR Sheep Insert per ewe for 5 days.
After insert removal, use standard flock breeding procedures to breed ewes at induced estrus.
Insertion
-
Insertion:
1. Avoid contact with skin by wearing protective gloves when handling inserts.
2. Only use the specially designed EAZI-BREED CIDR Sheep Insert Applicator for administration.
3. Restrain ewes appropriately prior to administration.
4. Wash the applicator in a non-irritating antiseptic solution, and then apply a veterinary obstetrical lubricant to the end
of the applicator.
5. Push the tail end of the EAZI-BREED CIDR Sheep Insert into the applicator taking care to assure the tail is extending upward through the slot of the applicator and is pointed away rom the handle.
6. Fold the wings of the EAZI-BREED CIDR Sheep Insert to make it longer and continue to advance the insert into the applicator
until it is fully seated with only the tips of the wings protruding from the end of the applicator (see Figure 1).
7. Lubricate the protruding tips of the wings of the EAZI-BREED CIDR Sheep Insert with veterinary obstetrical lubricant.
8. Clean the exterior of the vulva with disposable tissue.
9. Open the lips of the vulva and gently place the loaded applicator through the vulva. The slot in the applicator
should face down (see Figure 2).
10. Once the loaded applicator is past the vulva slope the applicator slightly upwards (35 - 45° angle) by lowering the
handle, and then forward, without forcing, until the applicator is fully inserted or resistance is felt (see Figure 3).
11. Squeeze the finger grips within the handle of the applicator to deposit the EAZI-BREED CIDR Sheep Insert in the anterior
vagina (see Figure 4) and then pull the applicator backwards to remove it from the vagina.
12. With the EAZI-BREED CIDR Sheep Insert correctly placed, with the wings open in the anterior portion of the vagina, the
tail of the insert should be visible, pointing downward from the vulva of the ewe.
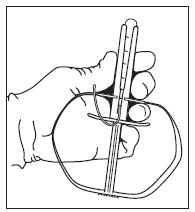
Figure 1
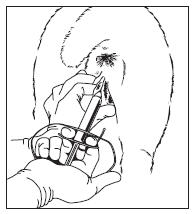
Figure 2
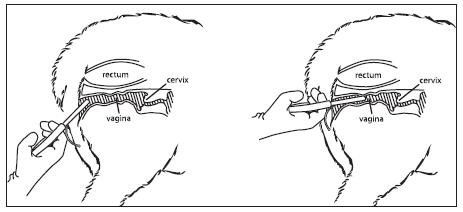
Figure 3
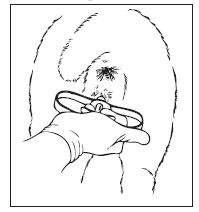
Figure 4
Removal
-
Removal:
1. Remove EAZI-BREED CIDR Sheep Inserts by pulling, gently but firmly, on the protruding nylon tail.
2. EAZI-BREED CIDR Sheep Inserts may reverse direction within the vagina; therefore, if the nylon tail of the insert is not visible on the day of removal, check the vagina to determine if an insert is present.
3. Used (removed) EAZI-BREED CIDR Sheep Inserts must be stored in a sealable container until disposed. Sealed bag/container with used EAZI-BREED CIDR Sheep Inserts must be properly disposed in accordance with applicable local, state and Federal regulations.
-
-
PRINCIPAL DISPLAY PANEL
EAZI-BREED™
CIDR®
(progesterone) Sheep Insert
NET CONTENTS: 20 EAZI-BREED CIDR Sheep Inserts per bag
Each EAZI-BREED CIDR Sheep Insert contains 0.3 gram of
progesterone in molded silicone over a flexible nylon spine.
Attached to each EAZI-BREED CIDR Sheep Insert is a nylon tail.
NADA 141-302, Approved by FDA

Label Eazi-Breed Sheep
-
INGREDIENTS AND APPEARANCE
EAZI-BREED CIDR SHEEP INSERT
progesterone insert, extended releaseProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 54771-1533 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 0.3 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-1533-1 20 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141302 10/01/2009 Labeler - Zoetis Inc. (828851555) Establishment Name Address ID/FEI Business Operations DEC International (NZ) Limited 595014127 MANUFACTURE Establishment Name Address ID/FEI Business Operations Zhejiang Shenzhou Pharmaceutical Co., Ltd. 527042153 API MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.