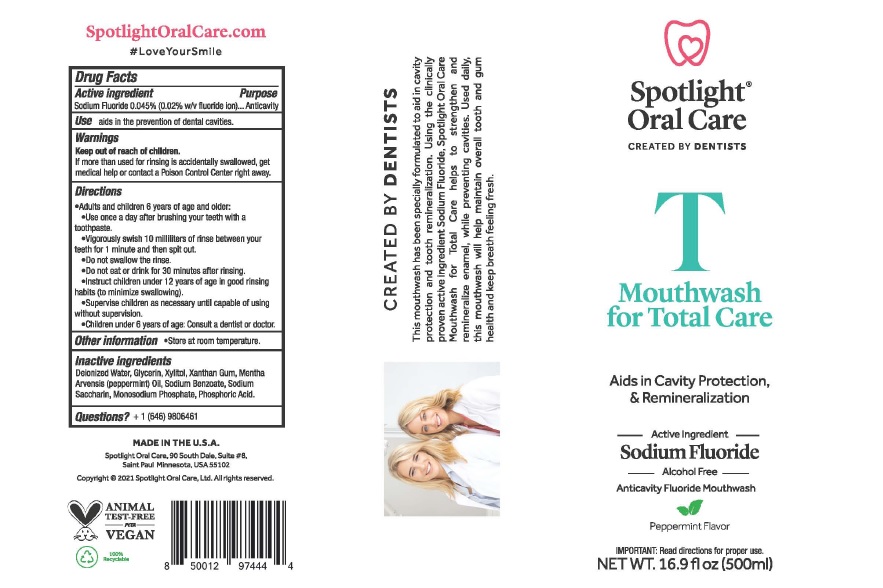
Spotlight ® Oral Care Anticavity Mouthwash for Total Care
Mouthwash for Total Care by
Drug Labeling and Warnings
Mouthwash for Total Care by is a Otc medication manufactured, distributed, or labeled by Oral Spotlight Care Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MOUTHWASH FOR TOTAL CARE- sodium fluoride liquid
Oral Spotlight Care Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Spotlight® Oral Care Anticavity Mouthwash for Total Care
Directions
Adults and children 6 years of age and older:
Use once a day after brushing your teeth with a toothpaste.
Vigorously swish 10 milliliters (2 teaspoonfuls) of rinse between your
teeth for 1 minute and then spit out.
Do not swallow the rinse.
Do not eat or drink for 30 minutes after rinsing.
Instruct children under 12 years of age in good rinsing
habits (to minimize swallowing).
Supervise children as necessary until capable of using
without supervision
Children under 6 years of age: Consult a dentist or doctor
Inactive ingredients
Deionized Water, Glycerin, Xylitol, Xanthan Gum, Mentha
Arvensis (peppermint) Oil, Sodium Benzoate, Sodium
Saccharin, Monosodium Phosphate, Phosphoric Acid.
Product Label
Spotlight®
Oral Care
CREATED BY DENTISTS
T
Mouthwash
for Total Care
Aids in Cavity Protection,
& Remineralization
-- Active Ingredient --
Sodium Fluoride
--- Alcohol Free --
Anticavity Fuoride Mouthwash
Peppermint Flavor
IMPORTANT: Read directions for proper use.
NET WT. 16.9 fl oz (500mL)
MADE IN THE USA
Spotlight Oral Care, 90 South Dale, Suite #8,
Saint Paul, Minnesota, USA 55102
res
| MOUTHWASH FOR TOTAL CARE
sodium fluoride liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Oral Spotlight Care Inc (117405870) |
| Registrant - Oral Spotlight Care Inc (117405870) |