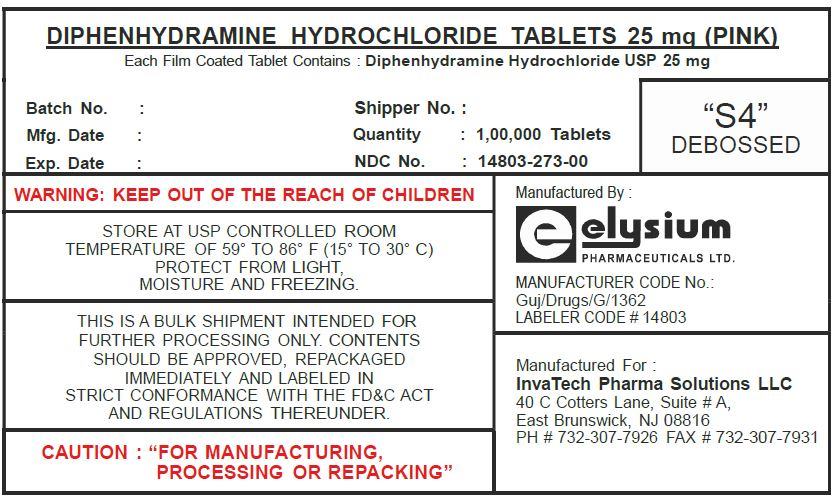
Diphenhydramine Hydrochloride
Diphenhydramine hydrochloride by
Drug Labeling and Warnings
Diphenhydramine hydrochloride by is a Otc medication manufactured, distributed, or labeled by Elysium Pharmaceuticals Ltd, Elysium Pharmaceuticals Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIPHENHYDRAMINE HYDROCHLORIDE- diphenhydramine hydrochloride tablet, film coated
Elysium Pharmaceuticals Ltd
----------
Diphenhydramine Hydrochloride
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Elysium Pharmaceuticals Ltd (915664486) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Elysium Pharmaceuticals Ltd. | 915664486 | manufacture(14803-273) , analysis(14803-273) | |
Revised: 1/2026
Document Id: 487a7d84-8594-361b-e063-6294a90a13e1
Set id: 131977ed-f1cb-1378-e063-6394a90ae815
Version: 4
Effective Time: 20260115