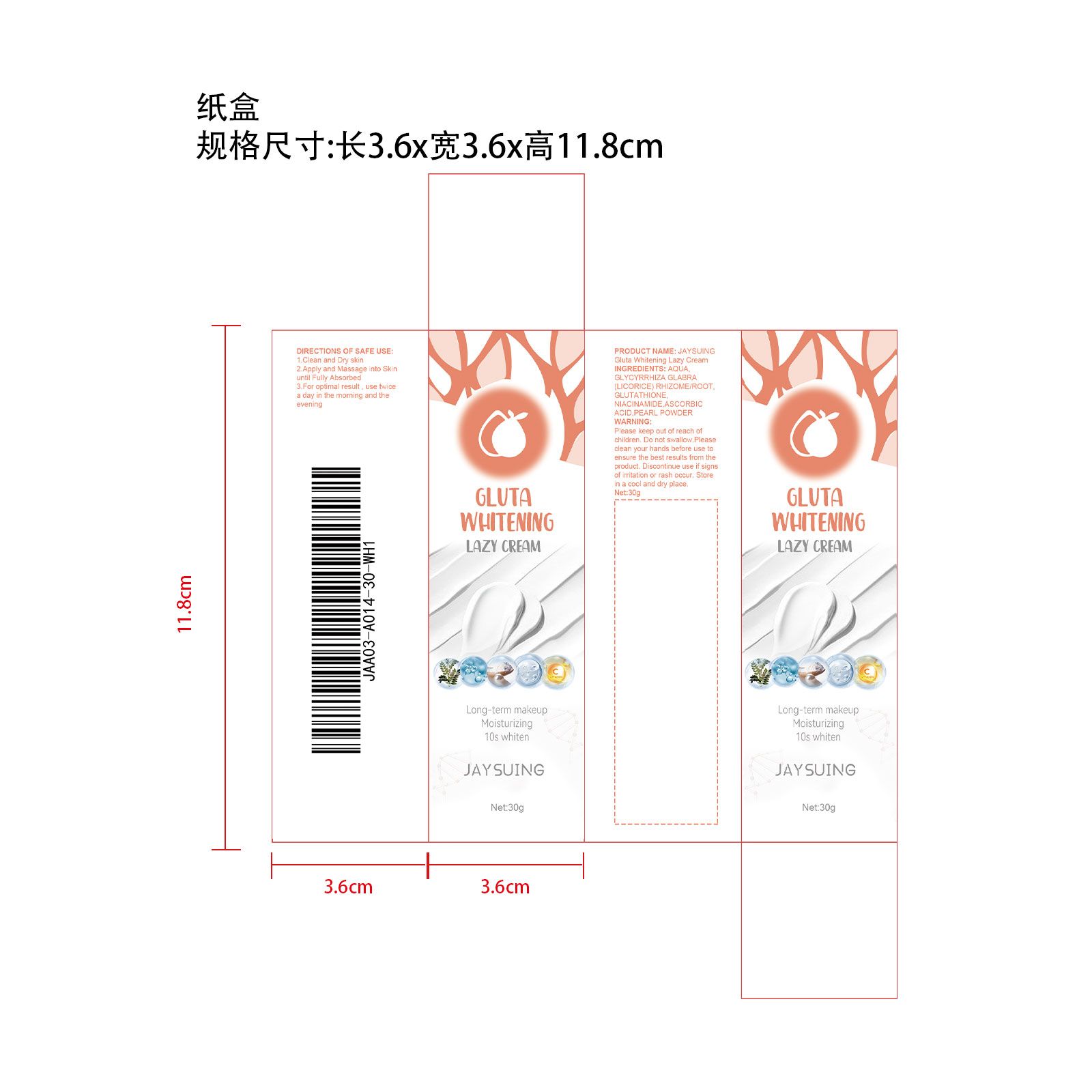
JAYSUING Gluta Whitening Lazy Cream
Gluta Whitening Lazy Cream by
Drug Labeling and Warnings
Gluta Whitening Lazy Cream by is a Otc medication manufactured, distributed, or labeled by Shantou Youjia E-Commerce Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GLUTA WHITENING LAZY CREAM- gluta whitening lazy cream cream
Shantou Youjia E-Commerce Co., Ltd.
----------
JAYSUING Gluta Whitening Lazy Cream
| GLUTA WHITENING LAZY CREAM
gluta whitening lazy cream cream |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Shantou Youjia E-Commerce Co., Ltd. (711173127) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shantou Youjia E-Commerce Co., Ltd. | 711173127 | label(84067-045) | |
Revised: 3/2024
Document Id: 13212eea-5462-9ab4-e063-6294a90a7b89
Set id: 13212eea-5461-9ab4-e063-6294a90a7b89
Version: 1
Effective Time: 20240308