WART FLAT- wart flat liquid
ShantouYoujiaE-CommerceCo.,Ltd.
----------
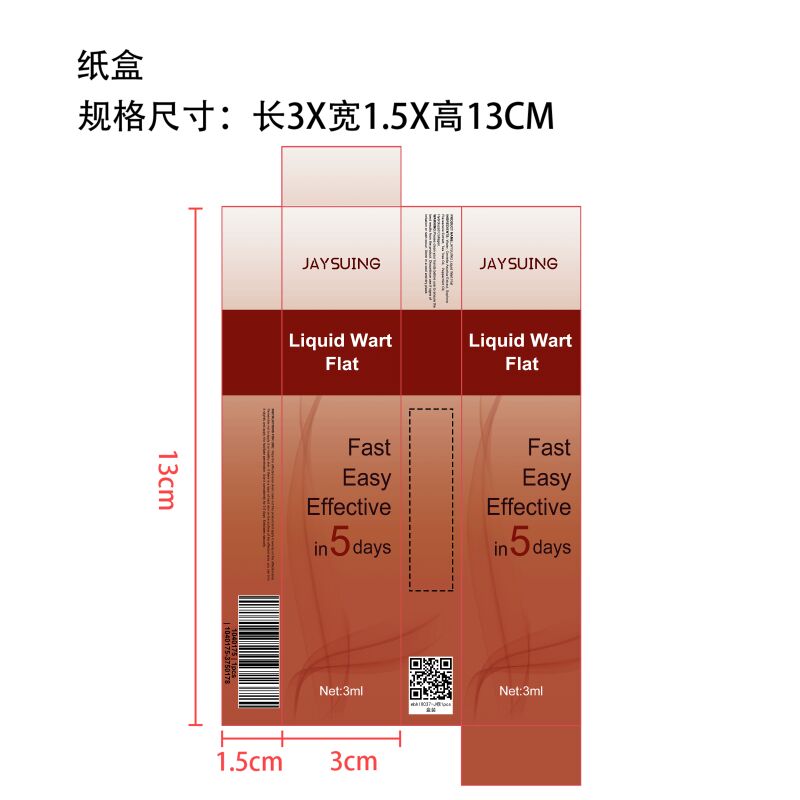
JAYSUING Liquid Wart Flat
WART FLAT
wart flat liquid |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 84067-053 |
| Route of Administration | CUTANEOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) | MENTHA PIPERITA | 0.3 mg in 3 mL |
| GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) (GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) - UNII:0K9R94573C) | GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) | 0.3 mg in 3 mL |
| CENTELLA ASIATICA (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) | CENTELLA ASIATICA | 0.3 mg in 3 mL |
| MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) (MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL - UNII:VIF565UC2G) | MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL | 0.3 mg in 3 mL |
| SOPHORA FLAVESCENS WHOLE (UNII: X8KX602M5L) (SOPHORA FLAVESCENS WHOLE - UNII:X8KX602M5L) | SOPHORA FLAVESCENS WHOLE | 0.3 mg in 3 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| WATER (UNII: 059QF0KO0R) | 0.9 mL in 3 mL |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 84067-053-01 | 3 mL in 1 BOX; Type 0: Not a Combination Product | 02/01/2024 | 12/31/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Export only | | 02/01/2024 | 12/31/2024 |
|
| Labeler - ShantouYoujiaE-CommerceCo.,Ltd.
(711173127)
|
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| ShantouYoujiaE-CommerceCo.,Ltd. | | 711173127 | label(84067-053) |