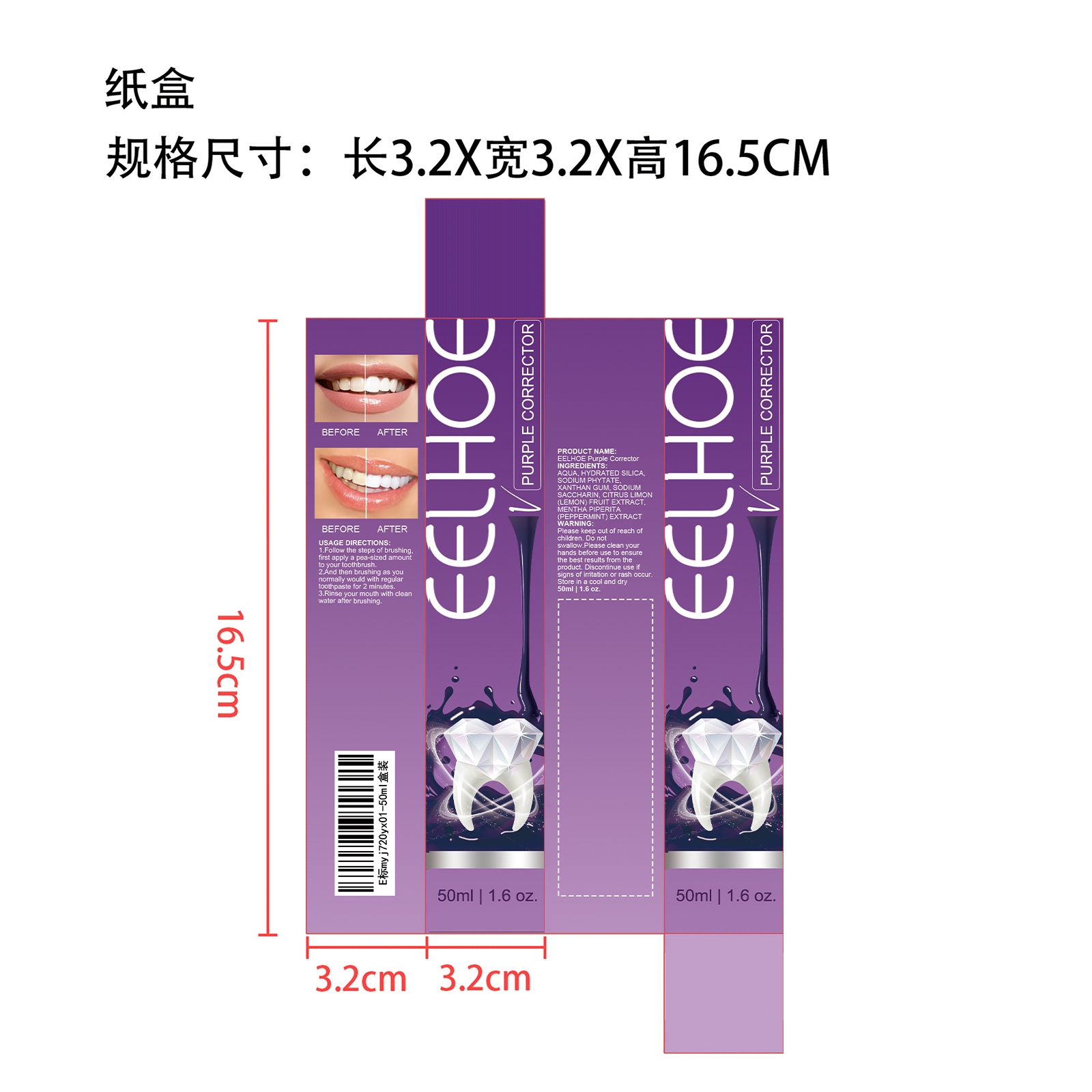
Purple Corrector by ShantouYoujiaE-CommerceCo.,Ltd. EELHOE Purple Corrector
Purple Corrector by
Drug Labeling and Warnings
Purple Corrector by is a Otc medication manufactured, distributed, or labeled by ShantouYoujiaE-CommerceCo.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PURPLE CORRECTOR- purple corrector paste, dentifrice
ShantouYoujiaE-CommerceCo.,Ltd.
----------
EELHOE Purple Corrector
| PURPLE CORRECTOR
purple corrector paste, dentifrice |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - ShantouYoujiaE-CommerceCo.,Ltd. (711173127) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ShantouYoujiaE-CommerceCo.,Ltd. | 711173127 | label(84067-105) | |
Revised: 3/2024
Document Id: 13328427-f676-593d-e063-6294a90a1f0d
Set id: 13328427-f675-593d-e063-6294a90a1f0d
Version: 1
Effective Time: 20240308