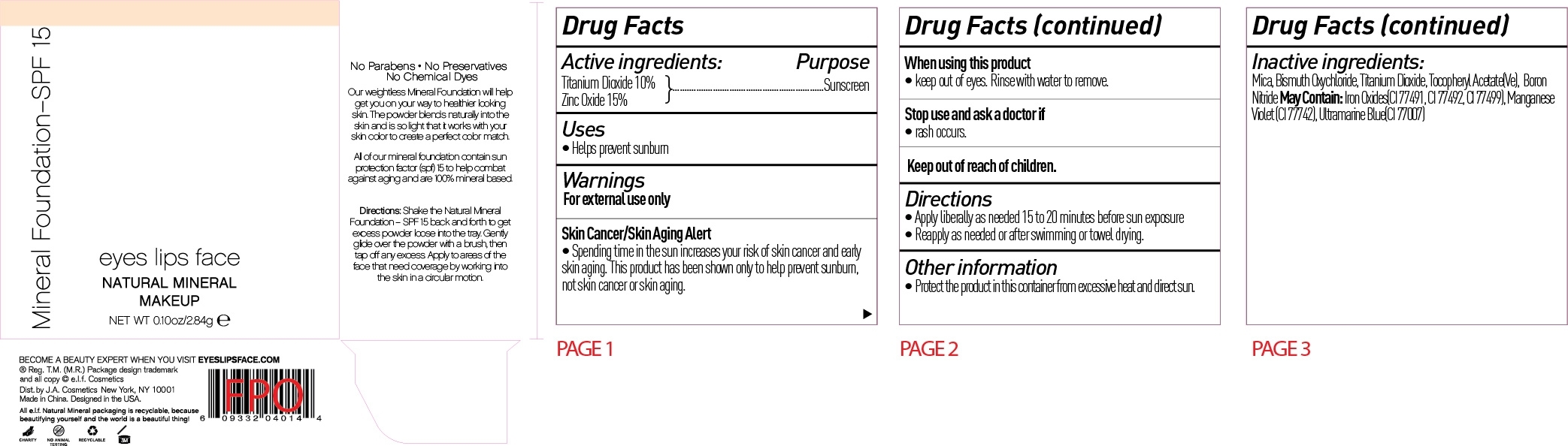
ELF Mineral Foundation SPF 15 by J. A. Cosmetics U.S. INC / Shanghai J. A. Cosmetics Trading CO., LTD. Drug Fact
ELF Mineral Foundation SPF 15 by
Drug Labeling and Warnings
ELF Mineral Foundation SPF 15 by is a Otc medication manufactured, distributed, or labeled by J. A. Cosmetics U.S. INC, Shanghai J. A. Cosmetics Trading CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ELF MINERAL FOUNDATION SPF 15 - titanium dioxide powder
J. A. Cosmetics U.S. INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Fact
Warning:
For external use only
Skin Cancer/Skin Aging Alert:
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown to only help prevent sunburn, not skin cancer and skin aging.
Directions:
Apply liberally as needed 15 to 20 minutes before sun exposure
Reapply as needed or after swimming or towel drying
Shake the Nature Mineral Foundation SPF 15 back and forth to get excess powder loose onto the tray. Gently glide over the powder with the Total Face Brush to grab the powder, bring your brush to the cap to work in the powder to the brush, then tap off any excess. Apply to the areas of the face that need coverage by working into the skin in a circular motion.
| ELF MINERAL FOUNDATION SPF 15
titanium dioxide powder |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - J. A. Cosmetics U.S. INC (186705047) |