ANTIBACTERIAL HAND SANITIZER Peach Scented
Vivitar ANTIBACTERIAL HAND SANITIZER Peach Scented by
Drug Labeling and Warnings
Vivitar ANTIBACTERIAL HAND SANITIZER Peach Scented by is a Otc medication manufactured, distributed, or labeled by Sakar International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VIVITAR ANTIBACTERIAL HAND SANITIZER PEACH SCENTED- ethyl alcohol gel
Sakar International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
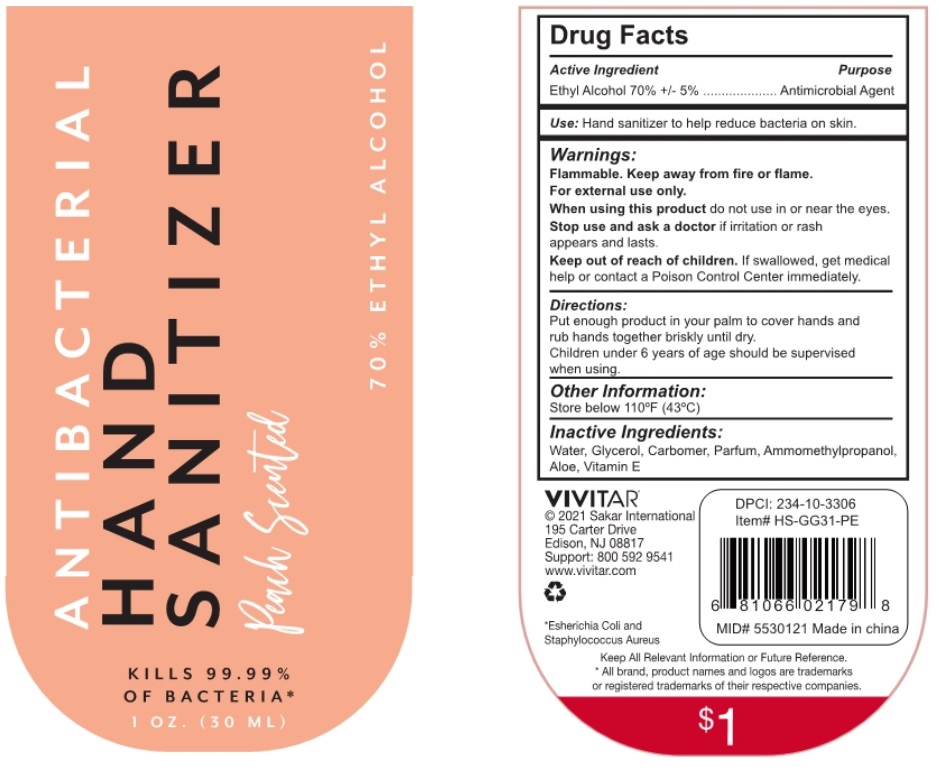
ANTIBACTERIAL HAND SANITIZER Peach Scented
Warnings:
Flammable. Keep away from fire or flame.
For external use only.
When using this product do not use in or near the eyes.
Stop use and ask a doctor if irritation or rash appears and lasts.
Directions:
Put enough product in your palm to cover hands and rub hands together briskly until dry.
Children under 6 years of age should be supervised when using.
Peach Scented
KILLS 99.99% OF BACTERIA*
70% ETHYL ALCOHOL
VIVITAR®
© 2021 Sakar International
195 Carter Drive
Edison, NJ 08817
Support: 800 592 9541
www.vivitar.com
Made in china
*Escherichia Coli and Staphylococcus Aureus
Keep All Relevant Information for Future Reference.
* All brand, product names and logos are trademarks or registered trademarks of their respective companies.
| VIVITAR ANTIBACTERIAL HAND SANITIZER PEACH SCENTED
ethyl alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Sakar International, Inc. (086413028) |