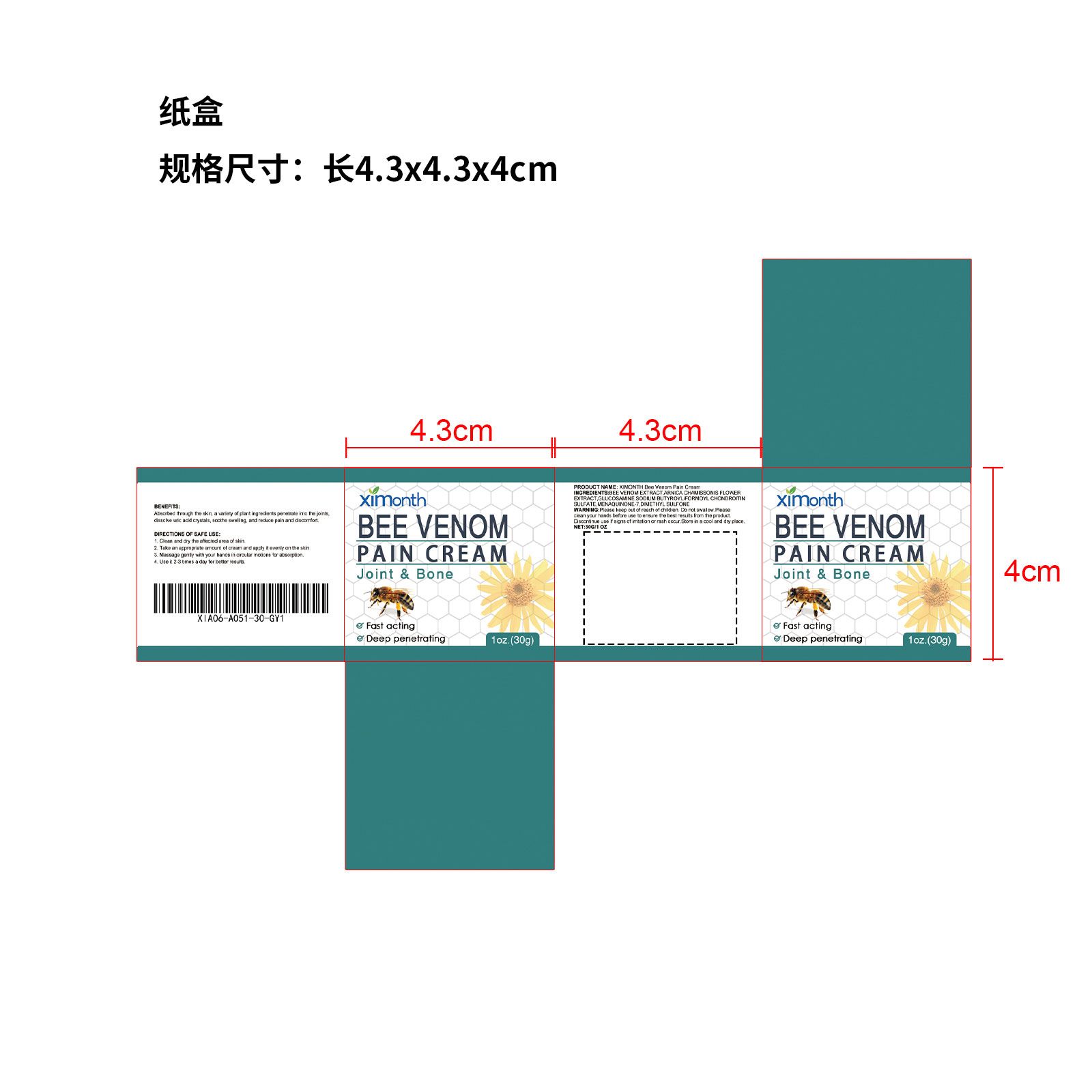
XIMONTH Bee Venom Pain Cream
Bee Venom Pain Cream by
Drug Labeling and Warnings
Bee Venom Pain Cream by is a Otc medication manufactured, distributed, or labeled by Shantou Youjia E-Commerce Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BEE VENOM PAIN CREAM- bee venom pain cream cream
Shantou Youjia E-Commerce Co., Ltd.
----------
XIMONTH Bee Venom Pain Cream
| BEE VENOM PAIN CREAM
bee venom pain cream cream |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Shantou Youjia E-Commerce Co., Ltd. (711173127) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shantou Youjia E-Commerce Co., Ltd. | 711173127 | label(84067-063) | |
Revised: 3/2024
Document Id: 135abbc5-0565-11cd-e063-6394a90a21bc
Set id: 135abbc5-0564-11cd-e063-6394a90a21bc
Version: 1
Effective Time: 20240311