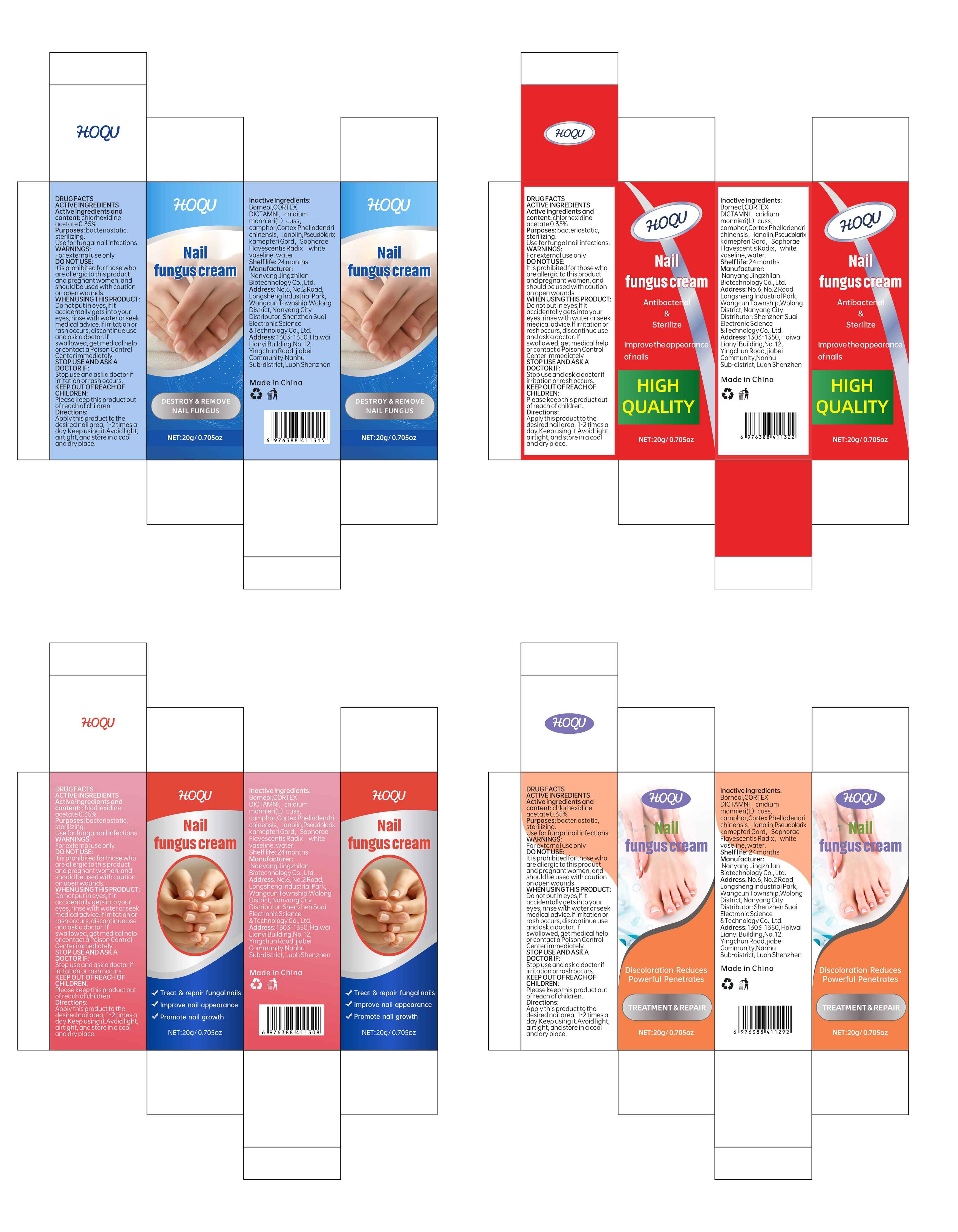
HOQU Nail fungus cream by Shenzhen Suai Electronic Science & Technology Co., Ltd. 84095-002 下架
HOQU Nail fungus cream by
Drug Labeling and Warnings
HOQU Nail fungus cream by is a Otc medication manufactured, distributed, or labeled by Shenzhen Suai Electronic Science & Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HOQU NAIL FUNGUS CREAM- nail fungus cream paste
Shenzhen Suai Electronic Science & Technology Co., Ltd.
----------
84095-002 下架
Do not use
lt is prohibited for those who are allergic to this product and pregnant women, and should be used with caution on open wounds.
When Using
Do not put in eyes,lf it accidentally gets into your eyes, rinse with water or seek medical advice.lf irritation or rash occurs, discontinue use and ask a doctor. lf swallowed, get medical help or contact a Poison Control Center immediately
Directions
Apply this product to the desired nail area, 1-2 times a day.Keep using it.Avoid light, airtight, and store in a cool and dry place.
| HOQU NAIL FUNGUS CREAM
nail fungus cream paste |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Shenzhen Suai Electronic Science & Technology Co., Ltd. (444420123) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Suai Electronic Science & Technology Co., Ltd. | 444420123 | label(84095-002) , manufacture(84095-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.