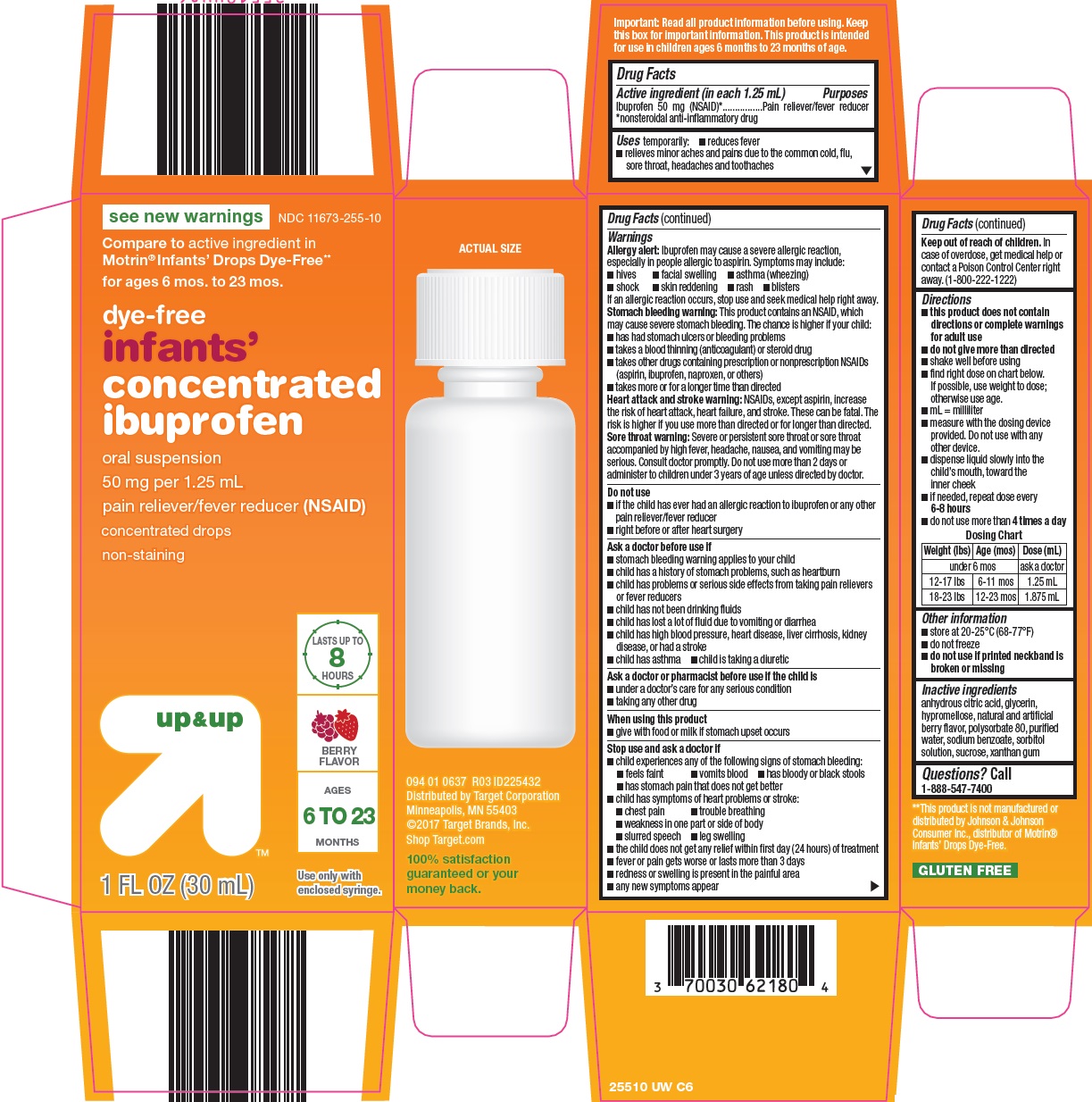
Target Corporation Concentrated Ibuprofen Oral Suspension Drug Facts
up and up dye free infants concentrated ibuprofen by
Drug Labeling and Warnings
up and up dye free infants concentrated ibuprofen by is a Otc medication manufactured, distributed, or labeled by Sixarp, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
UP AND UP DYE FREE INFANTS CONCENTRATED IBUPROFEN- ibuprofen suspension/ drops
Sixarp, LLC
----------
Target Corporation Concentrated Ibuprofen Oral Suspension Drug Facts
Uses
temporarily:
- reduces fever
- relieves minor aches and pains due to the common cold, flu, sore throat, headaches and toothaches
Warnings
Allergy alert:Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning:This product contains an NSAID, which may cause severe stomach bleeding.
The chance is higher if your child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed
Heart attack and stroke warning:NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Sore throat warning:Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
Do not use
- if the child has ever had an allergic reaction to ibuprofen or any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has problems or serious side effects from taking pain relievers or fever reducers
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child has high blood pressure, heart disease, liver cirrhosis, kidney disease, or had a stroke
- child has asthma
- child is taking a diuretic
Ask a doctor or pharmacist before use if the child is
- under a doctor’s care for any serious condition
- taking any other
Stop use and ask a doctor if
- child experiences any of the following signs of stomach bleeding:
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- child has symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- the child does not get any relief within first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise use age.
- mL = milliliter
- measure with the dosing device provided. Do not use with any other device.
- dispense liquid slowly into the child’s mouth, toward the inner cheek
- if needed, repeat dose every 6-8 hours
- do not use more than 4 times a day
- Dosing Chart
|
Weight (lbs) |
Age (mos) |
Dose (mL) |
|
under 6 mos |
ask a doctor |
|
|
12-17 lbs |
6-11 mos |
1.25 mL |
|
18-23 lbs |
12-23 mos |
1.875 mL |
Other information
- store at 20-25°C (68-77°F)
- do not freeze
- do not use if printed neckband is broken or missing
Inactive ingredients
anhydrous citric acid, glycerin, hypromellose, natural and artificial berry flavor, polysorbate 80, purified water, sodium benzoate, sorbitol solution, sucrose, xanthan gum
Principal Display Panel
see new warnings
Compare to active ingredient in Motrin® Infants' Drops Dye-Free
for ages 6 mos. to 23 mos.
dye-free
infants'
concentrated ibuprofen
oral suspension
50 mg per 1.25 mL
pain reliever/fever reducer (NSAID)
concentrated drops
non-staining
LASTS UP TO 8 HOURS
BERRY FLAVOR
AGES 6 TO 23 MONTHS
1 FL OZ (30 mL)
Use only with enclosed syringe.
| UP AND UP DYE FREE INFANTS CONCENTRATED IBUPROFEN
ibuprofen suspension/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sixarp, LLC (016329513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sixarp, LLC | 016329513 | manufacture(59368-003) , label(59368-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.