XOPENEX- levalbuterol hydrochloride solution
Xopenex by
Drug Labeling and Warnings
Xopenex by is a Prescription medication manufactured, distributed, or labeled by REMEDYREPACK INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XOPENEX ® Inhalation Solution safely and effectively. See full prescribing information for XOPENEX ® Inhalation Solution.
XOPENEX ® (levalbuterol hydrochloride) Inhalation Solution, for inhalation use
Initial U.S. Approval: 1999INDICATIONS AND USAGE
XOPENEX (levalbuterol hydrochloride) Inhalation Solution is a beta 2-adrenergic agonist indicated for:
- Treatment or prevention of bronchospasm in adults, adolescents, and children 6 years of age and older with reversible obstructive airway disease. ( 1)
DOSAGE AND ADMINISTRATION
- FOR ORAL INHALATION ONLY ( 2)
- Children 6-11 years old: 0.31 mg administered three times a day, by nebulization. Routine dosing should not exceed 0.63 mg three times a day. ( 2)
- Adults and Adolescents ≥12 years old: 0.63 mg administered three times a day, every 6 to 8 hours, by nebulization. The maximum recommended dose is 1.25 mg three times a day. ( 2)
- For use with a standard jet nebulizer (with a face mask or mouthpiece) connected to an air compressor. ( 2)
DOSAGE FORMS AND STRENGTHS
Inhalation Solution (unit-dose vial for nebulization): 0.31 mg/3 mL, 0.63 mg/3 mL and 1.25 mg/3 mL. ( 3)
CONTRAINDICATIONS
- Hypersensitivity to levalbuterol or racemic albuterol. ( 4)
WARNINGS AND PRECAUTIONS
- Life-threatening paradoxical bronchospasm may occur. Discontinue XOPENEX Inhalation Solution immediately and treat with alternative therapy. ( 5.1)
- Need for more doses of XOPENEX Inhalation Solution than usual may be a sign of deterioration of asthma and requires reevaluation of treatment. ( 5.2)
- XOPENEX Inhalation Solution is not a substitute for corticosteroids. ( 5.3)
- Cardiovascular effects may occur. Consider discontinuation of XOPENEX Inhalation Solution if these effects occur. Use with caution in patients with underlying cardiovascular disorders. ( 5.4)
- Excessive use may be fatal. Do not exceed recommended dose. ( 5.5)
- Immediate hypersensitivity reactions may occur. Discontinue XOPENEX Inhalation Solution immediately. ( 5.6)
- Hypokalemia and changes in blood glucose may occur. ( 5.7, 5.8)
ADVERSE REACTIONS
Most common adverse reactions are: palpitations, chest pain, tachycardia, headache, dizziness, tremor and nervousness. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Akorn, Inc. at 1-800-932-5676 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Other short-acting sympathomimetic aerosol bronchodilators and adrenergic drugs: May potentiate effect. ( 7.1)
- Beta-blockers: May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. Patients with asthma should not normally be treated with beta-blockers. ( 7.2)
- Diuretic: May worsen electrocardiographic changes or hypokalemia associated with diuretic may worsen. Consider monitoring potassium levels. ( 7.3)
- Digoxin: May decrease serum digoxin levels. Consider monitoring digoxin levels. ( 7.4)
- Monoamine oxidase inhibitors (MAOs) or tricyclic antidepressants: May potentiate effect of albuterol on the cardiovascular system. ( 7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm
5.2 Deterioration of Asthma
5.3 Use of Anti-Inflammatory Agents
5.4 Cardiovascular Effects
5.5 Do Not Exceed Recommended Dose
5.6 Immediate Hypersensitivity Reactions
5.7 Coexisting Conditions
5.8 Hypokalemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Short-Acting Bronchodilators
7.2 Beta-blockers
7.3 Diuretics
7.4 Digoxin
7.5 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
XOPENEX Inhalation Solution is for oral inhalation only. Administer by nebulization using with a standard jet nebulizer (with a face mask or mouthpiece) connected to an air compressor. Do not exceed recommended dose.
Children 6-11 years old: The recommended dosage of XOPENEX Inhalation Solution for patients 6-11 years old is 0.31 mg administered three times a day, by nebulization. Routine dosing should not exceed 0.63 mg three times a day.
Adults and Adolescents ≥12 years old: The recommended starting dosage of XOPENEX Inhalation Solution for patients 12 years of age and older is 0.63 mg administered three times a day, every 6 to 8 hours, by nebulization.
Patients 12 years of age and older with more severe asthma or patients who do not respond adequately to a dose of 0.63 mg of XOPENEX Inhalation Solution may benefit from a dosage of 1.25 mg three times a day.
Patients receiving the highest dose of XOPENEX Inhalation Solution should be monitored closely for adverse systemic effects, and the risks of such effects should be balanced against the potential for improved efficacy.
The use of XOPENEX Inhalation Solution can be continued as medically indicated to help control recurring bouts of bronchospasm. During this time, most patients gain optimal benefit from regular use of the inhalation solution.
If a previously effective dosage regimen fails to provide the usual response this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
The drug compatibility (physical and chemical), efficacy, and safety of XOPENEX Inhalation Solution when mixed with other drugs in a nebulizer have not been established.
The safety and efficacy of XOPENEX Inhalation Solution have been established in clinical trials when administered using the PARI LC Jet™ and PARI LC Plus™ nebulizers, and the PARI Master ® Dura-Neb ® 2000 and Dura-Neb ® 3000 compressors. The safety and efficacy of XOPENEX Inhalation Solution when administered using other nebulizer systems have not been established.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
XOPENEX Inhalation Solution is contraindicated in patients with a history of hypersensitivity to levalbuterol or racemic albuterol. Reactions have included urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema [see Warnings and Precautions ( 5.6) ].
-
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm
XOPENEX Inhalation Solution can produce paradoxical bronchospasm, which may be life-threatening. If paradoxical bronchospasm occurs, XOPENEX Inhalation Solution should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new vial.
5.2 Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of XOPENEX Inhalation Solution than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
5.3 Use of Anti-Inflammatory Agents
XOPENEX Inhalation Solution is not a substitute for corticosteroids. The use of beta-adrenergic agonist alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids, to the therapeutic regimen.
5.4 Cardiovascular Effects
XOPENEX Inhalation Solution, like other beta-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients, as measured by heart rate, blood pressure, and symptoms. Although such effects are uncommon after administration of XOPENEX Inhalation Solution at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the t-wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, XOPENEX Inhalation Solution, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.5 Do Not Exceed Recommended Dose
Do not exceed the recommended dose. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs in patients with asthma. The exact cause of death is unknown, but cardiac arrest following an unexpected development of a severe acute asthmatic crisis and subsequent hypoxia is suspected.
5.6 Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of levalbuterol or racemic albuterol. Reactions have included urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. The potential for hypersensitivity must be considered in the clinical evaluation of patients who experience immediate hypersensitivity reactions while receiving XOPENEX Inhalation Solution.
5.7 Coexisting Conditions
XOPENEX Inhalation Solution, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, hypertension, and cardiac arrhythmias; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Clinically significant changes in systolic and diastolic blood pressure have been seen in individual patients and could be expected to occur in some patients after the use of any beta-adrenergic bronchodilator.
Changes in blood glucose may occur. Large doses of intravenous racemic albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.8 Hypokalemia
As with other beta-adrenergic agonist medications, XOPENEX Inhalation Solution may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Paradoxical bronchospasm [ see Warnings and Precautions ( 5.1) ]
- Cardiovascular effects [ see Warnings and Precautions ( 5.4) ]
- Immediate hypersensitivity reactions [ see Warnings and Precautions ( 5.6) ]
- Hypokalemia [ see Warnings and Precautions ( 5.8) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of the drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults and Adolescents 12 Years of Age and Older:
Adverse reaction information concerning XOPENEX Inhalation Solution in adults and adolescents is derived from one 4-week, multicenter, randomized, double-blind, active-, and placebo-controlled trial in 362 patients with asthma 12 years of age and older. Adverse reactions reported in ≥2% of patients receiving XOPENEX Inhalation Solution or racemic albuterol and more frequently than in patients receiving placebo are listed in Table 1.
Table 1: Adverse Reactions Reported in a 4-Week, Controlled Clinical Trial in Adults and Adolescents ≥12 Years Old a One treatment group, racemic albuterol 1.25 mg, with 68 subjects is omitted.
Percent of Patients a Placebo
(n=75)XOPENEX
1.25 mg
(n=73)XOPENEX
0.63 mg
(n=72)Racemic albuterol
2.5 mg
(n=74)Body System Preferred Term Body as a Whole
Allergic reaction1.3 0 0 2.7 Flu syndrome 0 1.4 4.2 2.7 Accidental injury 0 2.7 0 0 Pain 1.3 1.4 2.8 2.7 Back pain 0 0 0 2.7 Cardiovascular System
Tachycardia0 2.7 2.8 2.7 Migraine 0 2.7 0 0 Digestive System
Dyspepsia1.3 2.7 1.4 1.4 Musculoskeletal System
Leg cramps1.3 2.7 0 1.4 Central Nervous System
Dizziness1.3 2.7 1.4 0 Hypertonia 0 0 0 2.7 Nervousness 0 9.6 2.8 8.1 Tremor 0 6.8 0 2.7 Anxiety 0 2.7 0 0 Respiratory System
Cough increased2.7 4.1 1.4 2.7 Infection viral 9.3 12.3 6.9 12.2 Rhinitis 2.7 2.7 11.1 6.8 Sinusitis 2.7 1.4 4.2 2.7 Turbinate edema 0 1.4 2.8 0 The incidence of certain systemic beta-adrenergic adverse reactions (e.g., tremor, nervousness) was slightly less in the XOPENEX Inhalation Solution 0.63 mg group compared with the other active treatment groups. The clinical significance of these small differences is unknown.
Changes in heart rate 15 minutes after drug administration and in plasma glucose and potassium 1 hour after drug administration on day 1 and day 29 were clinically comparable in the XOPENEX Inhalation Solution 1.25 mg and racemic albuterol 2.5 mg groups (see Table 2). Changes in heart rate and plasma glucose were slightly less in the XOPENEX Inhalation Solution 0.63 mg group compared with the other active treatment groups (see Table 2). The clinical significance of these small differences is unknown. After 4 weeks, effects on heart rate, plasma glucose, and plasma potassium were generally diminished compared with day 1 in all active treatment groups.
Table 2: Mean Changes from Baseline Heart Rate at 15 Minutes and Glucose and Potassium at 1 Hour after First Dose (Day 1) in Adults and Adolescents ≥12 Years Old Mean Changes (day 1) Heart Rate Glucose Potassium Treatment (bpm) (mg/dL) (mEq/L) XOPENEX 0.63 mg, n=72 2.4 4.6 -0.2 XOPENEX 1.25 mg, n=73 6.9 10.3 -0.3 Racemic albuterol 2.5 mg, n=74 5.7 8.2 -0.3 Placebo, n=75 -2.8 -0.2 -0.2 No other clinically relevant laboratory abnormalities related to administration of XOPENEX Inhalation Solution were observed in this study.
In the clinical trials, a slightly greater number of serious adverse events, discontinuations due to adverse events, and clinically significant ECG changes were reported in patients who received XOPENEX 1.25 mg compared with the other active treatment groups.
The following adverse reactions, considered potentially related to XOPENEX, occurred in less than 2% of the 292 subjects who received XOPENEX and more frequently than in patients who received placebo in any clinical trial:
Body as a Whole: chills, pain, chest pain Cardiovascular System: ECG abnormal, ECG change, hypertension, hypotension, syncope Digestive System: diarrhea, dry mouth, dry throat, dyspepsia, gastroenteritis, nausea Hemic and Lymphatic System: lymphadenopathy Musculoskeletal System: leg cramps, myalgia Nervous System: anxiety, hyperesthesia of the hand, insomnia, paresthesia, tremor Special Senses: eye itch The following reactions, considered potentially related to XOPENEX, occurred in less than 2% of the treated subjects but at a frequency less than in patients who received placebo: asthma exacerbation, cough increased, wheezing, sweating, and vomiting.
Pediatric Patients 6 to 11 Years of Age:
Adverse reaction information concerning XOPENEX Inhalation Solution in pediatric patients is derived from one 3-week, multicenter, randomized, double-blind, active-, and placebo-controlled trial in 316 pediatric patients 6 to 11 years of age. Adverse reactions reported in ≥2% of patients in any treatment group and more frequently than in patients receiving placebo are listed in Table 3.
Table 3: Most Frequently Reported Adverse Reactions (≥2% in Any Treatment Group) and Those Reported More Frequently Than in Placebo during the Double-Blind Period (ITT Population, 6-11 Years Old) Percent of Patients Racemic Racemic XOPENEX XOPENEX albuterol albuterol Body System Placebo 0.31 mg 0.63 mg 1.25 mg 2.5 mg Preferred Term (n=59) (n=66) (n=67) (n=64) (n=60) Body as a Whole
Abdominal pain3.4 0 1.5 3.1 6.7 Accidental injury 3.4 6.1 4.5 3.1 5.0 Asthenia 0 3.0 3.0 1.6 1.7 Fever 5.1 9.1 3.0 1.6 6.7 Headache 8.5 7.6 11.9 9.4 3.3 Pain 3.4 3.0 1.5 4.7 6.7 Viral infection 5.1 7.6 9.0 4.7 8.3 Digestive System
Diarrhea0 1.5 6.0 1.6 0 Hemic and Lymphatic
Lymphadenopathy0 3.0 0 1.6 0 Musculoskeletal System
Myalgia0 0 1.5 1.6 3.3 Respiratory System
Asthma5.1 9.1 9.0 6.3 10.0 Pharyngitis 6.8 3.0 10.4 0 6.7 Rhinitis 1.7 6.1 10.4 3.1 5.0 Skin and Appendages
Eczema0 0 0 0 3.3 Rash 0 0 7.5 1.6 0 Urticaria 0 0 3.0 0 0 Special Senses
Otitis media1.7 0 0 0 3.3 Note: Subjects may have more than one adverse event per body system and preferred term.
Changes in heart rate, plasma glucose, and serum potassium are shown in Table 4. The clinical significance of these small differences is unknown.
Table 4: Mean Changes from Baseline Heart Rate at 30 Minutes and Glucose and Potassium at 1 Hour after First Dose (Day 1) and Last Dose (Day 21) in Children 6-11 Years Old Mean Changes (Day 1) Heart Rate Glucose Potassium Treatment (bpm) (mg/dL) (mEq/L) XOPENEX 0.31 mg, n=66 0.8 4.9 -0.31 XOPENEX 0.63 mg, n=67 6.7 5.2 -0.36 Racemic albuterol 1.25 mg, n=64 6.4 8.0 -0.27 Racemic albuterol 2.5 mg, n=60 10.9 10.8 -0.56 Placebo, n=59 -1.8 0.6 -0.05 Table 4: Mean Changes from Baseline Heart Rate at 30 Minutes and Glucose and Potassium at 1 Hour after First Dose (Day 1) and Last Dose (Day 21) in Children 6-11 Years Old (continued) Mean Changes (Day 21) Heart Rate Glucose Potassium Treatment (bpm) (mg/dL) (mEq/L) XOPENEX 0.31 mg, n=60 0 2.6 -0.32 XOPENEX 0.63 mg, n=66 3.8 5.8 -0.34 Racemic albuterol 1.25 mg, n=62 5.8 1.7 -0.18 Racemic albuterol 2.5 mg, n=54 5.7 11.8 -0.26 Placebo, n=55 -1.7 1.1 -0.04 6.2 Post-marketing Experience
In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been observed in postapproval use of XOPENEX Inhalation Solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to their seriousness, their frequency of reporting, or their likely beta-mediated mechanism: angioedema, anaphylaxis, arrhythmias (including atrial fibrillation, supraventricular tachycardia, extrasystoles), asthma, chest pain, cough increased, dysphonia, dyspnea, gastrooesophageal reflux disease (GERD), metabolic acidosis, nausea, nervousness, rash, tachycardia, tremor, urticaria.
In addition, XOPENEX Inhalation Solution, like other sympathomimetic agents, can cause adverse reactions such as hypertension, angina, vertigo, central nervous system stimulation, sleeplessness, headache, and drying or irritation of the oropharynx.
-
7 DRUG INTERACTIONS
7.1 Short-Acting Bronchodilators
Avoid concomitant use of other short-acting sympathomimetic bronchodilators or epinephrine in patients being treated with XOPENEX Inhalation Solution. If additional adrenergic drugs are to be administered by any route, they should be used with caution to avoid deleterious cardiovascular effects.
7.2 Beta-blockers
Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-adrenergic agonists such as XOPENEX Inhalation Solution, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers should be considered, although they should be administered with caution.
7.3 Diuretics
The ECG changes or hypokalemia that may result from the administration of non-potassium-sparing diuretics (such as loop and thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non-potassium-sparing diuretics. Consider monitoring potassium levels.
7.4 Digoxin
Mean decreases of 16% and 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of racemic albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving XOPENEX Inhalation Solution and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and XOPENEX Inhalation Solution.
7.5 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
XOPENEX Inhalation Solution should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of levalbuterol on the vascular system may be potentiated. Consider alternative therapy in patients taking MAO inhibitors or tricyclic antidepressants.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C.
There are no adequate and well-controlled studies of XOPENEX Inhalation Solution in pregnant women. Because animal reproduction studies are not always predictive of human response, XOPENEX Inhalation Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been reported in newborns of women treated with racemic albuterol which contains the levalbuterol isomer (active drug substance of XOPENEX Inhalation Solution). However, since multiple medications were taken during some of the pregnancies and there was no consistent pattern of anomalies, it was not possible to establish a relationship between racemic albuterol use and the occurrence of these congenital anomalies.
In animal studies, oral administration of levalbuterol HCl to pregnant New Zealand White rabbits found no evidence of teratogenicity at doses up to 25 mg/kg/day (approximately 108 times the maximum recommended daily inhalation [MRDI] dose of levalbuterol HCl for adults on a mg/m 2 basis).
However, other studies demonstrated that racemic albuterol sulfate was teratogenic in mice and rabbits at doses comparable to the human therapeutic range. Pregnant mice administered racemic albuterol sulfate subcutaneously had a dose-related increased incidence of cleft palate in their fetuses (4.5% of fetuses at 0.25 mg/kg/day or greater, corresponding to approximately 0.3 times the MRDI dose, 9.3% of fetuses at 2.5 mg/kg/day, approximately 3 times the MRDI dose of levalbuterol HCl for adults on a mg/m 2 basis). The drug did not induce cleft palate formation when administered subcutaneously at a dose of 0.025 mg/ kg/day (approximately 0.03 times the MRDI dose of levalbuterol HCl for adults on a mg/m 2 basis). In addition, oral administration of racemic albuterol sulfate to pregnant rabbits resulted in an increased incidence of cranioschisis in fetuses (approximately 215 times the MRDI dose of levalbuterol HCl for adults on a mg/m 2 basis).
8.2 Labor and Delivery
Because of the potential for beta-adrenergic agonists to interfere with uterine contractility, the use of XOPENEX Inhalation Solution for the treatment of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
XOPENEX Inhalation Solution has not been approved for the management of preterm labor. The benefit:risk ratio when levalbuterol HCl is administered for tocolysis has not been established. Serious adverse reactions, including maternal pulmonary edema, have been reported during or following treatment of premature labor with beta 2-agonists, including racemic albuterol.
8.3 Nursing Mothers
Plasma concentrations of levalbuterol after inhalation of therapeutic doses are very low in humans. It is not known whether levalbuterol is excreted in human milk.
Because of the potential for tumorigenicity shown for racemic albuterol in animal studies and the lack of experience with the use of XOPENEX Inhalation Solution by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution should be exercised when XOPENEX Inhalation Solution is administered to a nursing woman.
8.4 Pediatric Use
Pediatric Patients 6 Years of Age and Older
The safety and efficacy of XOPENEX Inhalation Solution have been established in pediatric patients 6 years of age and older in an adequate and well-controlled clinical trial [see Adverse Reactions ( 6) and Clinical Studies ( 14) ].
Pediatric Patients less than 6 Years of Age
XOPENEX Inhalation Solution is not indicated for pediatric patients less than 6 years of age.
Clinical trials with XOPENEX Inhalation Solution in this age group failed to meet the primary efficacy endpoint and demonstrated an increased number of asthma-related adverse reactions following chronic XOPENEX treatment.
XOPENEX Inhalation Solution was studied in 379 pediatric patients less than 6 years of age with asthma or reactive airway disease - (291 patients 2 to 5 years of age, and 88 patients from birth to less than 2 years of age). Efficacy and safety data for XOPENEX Inhalation Solution in this age group are primarily available from one 3-week, multicenter, randomized, double-blind, active and placebo-controlled study (Study 1) in 211 pediatric patients between the ages of 2 and 5 years, of whom 119 received XOPENEX Inhalation Solution. Over the 3 week treatment period, there were no significant treatment differences in the Pediatric Asthma Questionnaire (PAQ) total score between groups receiving XOPENEX Inhalation Solution 0.31 mg, XOPENEX Inhalation Solution 0.63 mg, racemic albuterol, and placebo. Additional safety data following chronic dosing is available from a 4-week, multicenter, randomized, modified-blind, placebo-controlled study (Study 2) of 196 patients between the ages of birth and 3 years, of whom 63 received open-label XOPENEX Inhalation Solution. In these two studies, treatment-emergent asthma exacerbations or asthma-related adverse reactions and treatment discontinuations due to asthma occurred at a higher frequency in XOPENEX Inhalation-treated subjects compared to control ( Table 5). Other adverse reactions were consistent with those observed in the clinical trial population of patients 6 years of age and older [see Adverse Reactions ( 6.1)].
Table 5: Asthma-related Adverse Reactions in 3- and 4-Week Clinical Trials in Children Birth to <6 Years of Age *Asthma exacerbation defined as worsening of asthma symptoms or pulmonary function that required any of the following: emergency department visit, hospitalization, therapeutic intervention with oral or parenteral steroids, unscheduled clinic visit to treat acute asthma symptoms
**Includes the following Preferred Terms (whether considered by the investigator to be related or unrelated to drug): asthma, cough, hypoxia, status asthmaticus, tachypnea
Asthma
Exacerbations*
n (%)Treatment
Discontinuations
due to Asthma
n (%)Asthma-related
Adverse Reactions**
n (%)Study 1
XOPENEX 0.31 mg, n=586 (10) 4 (7) — XOPENEX 0.63 mg, n=51 7 (14) 6 (12) — Racemic albuterol, n=52 3 (6) 2 (4) — Placebo, n=50 2 (4) 2 (4) — Study 2
XOPENEX 0.31 mg, n=63— 2 (3) 6 (10) Levalbuterol HFA inhalation aerosol, n=65 — 1 (2) 8 (12) Placebo, n=68 — 0 3 (4) 8.5 Geriatric Use
Clinical studies of XOPENEX Inhalation Solution did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Only 5 patients 65 years of age and older were treated with XOPENEX Inhalation Solution in a 4-week clinical study [see Clinical Pharmacology ( 12) and Clinical Studies ( 14) ] (n=2 for 0.63 mg and n=3 for 1.25 mg). In these patients, bronchodilation was observed after the first dose on day 1 and after 4 weeks of treatment. In general, patients 65 years of age and older should be started at a dose of 0.63 mg of XOPENEX Inhalation Solution. If clinically warranted due to insufficient bronchodilator response, the dose of XOPENEX Inhalation Solution may be increased in elderly patients as tolerated, in conjunction with frequent clinical and laboratory monitoring, to the maximum recommended daily dose [see Dosage and Administration ( 2) ].
8.6 Renal Impairment
Albuterol is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
10 OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic receptor stimulation and/or occurrence or exaggeration of any of the symptoms listed under Adverse Reactions ( 6) , e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min., arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and sleeplessness. Hypokalemia also may occur. As with all sympathomimetic medications, cardiac arrest and even death may be associated with the abuse of XOPENEX Inhalation Solution. Treatment consists of discontinuation of XOPENEX Inhalation Solution together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of XOPENEX Inhalation Solution.
-
11 DESCRIPTION
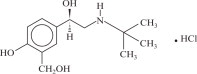
XOPENEX Inhalation Solution is a sterile, clear, colorless, preservative-free solution of the hydrochloride salt of levalbuterol, the (R)-enantiomer of the drug substance racemic albuterol. Levalbuterol HCl is a relatively selective beta 2-adrenergic receptor agonist [see Clinical Pharmacology ( 12) ]. The chemical name for levalbuterol HCl is (R)-α 1-[[(1,1-dimethylethyl) amino]methyl]-4-hydroxy-1,3-benzenedimethanol hydrochloride, and its established chemical structure is as follows:
The molecular weight of levalbuterol HCl is 275.8, and its empirical formula is C 13H 21NO 3HCl. It is a white to off-white, crystalline solid, with a melting point of approximately 187°C and solubility of approximately 180 mg/mL in water.
Levalbuterol HCl is the USAN modified name for (R)-albuterol HCl in the United States.
XOPENEX Inhalation Solution is supplied in unit-dose vials and requires no dilution before administration by nebulization. Each 3 mL unit-dose vial contains 0.31 mg of levalbuterol (as 0.36 mg of levalbuterol HCl) or 0.63 mg of levalbuterol (as 0.73 mg of levalbuterol HCl) or 1.25 mg of levalbuterol (as 1.44 mg of levalbuterol HCl), sodium chloride to adjust tonicity, and sulfuric acid to adjust the pH to 4.0 (3.3 to 4.5).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Activation of beta 2-adrenergic receptors on airway smooth muscle leads to the activation of adenylate cyclase and to an increase in the intracellular concentration of cyclic-3′, 5′ adenosine monophosphate (cyclic AMP). The increase in cyclic AMP is associated with the activation of protein kinase A, which in turn inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in muscle relaxation. Levalbuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway. Levalbuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. While it is recognized that beta 2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there are beta-receptors in the human heart, 10% to 50% of which are beta 2-adrenergic receptors. The precise function of these receptors has not been established [see Warnings and Precautions ( 5.4) ]. However, all beta-adrenergic agonist drugs can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
12.2 Pharmacodynamics
Adults and Adolescents ≥12 Years Old
In a randomized, double-blind, placebo-controlled, cross-over study, 20 adults with mild-tomoderate asthma received single doses of XOPENEX Inhalation Solution (0.31 mg, 0.63 mg, and 1.25 mg) and racemic albuterol sulfate inhalation solution (2.5 mg). All doses of active treatment produced a significantly greater degree of bronchodilation (as measured by percent change from pre-dose mean FEV 1) than placebo, and there were no significant differences between any of the active treatment arms. The bronchodilator responses to 1.25 mg of XOPENEX Inhalation Solution and 2.5 mg of racemic albuterol sulfate inhalation solution were clinically comparable over the 6-hour evaluation period, except for a slightly longer duration of action (>15% increase in FEV 1 from baseline) after administration of 1.25 mg of XOPENEX Inhalation Solution. Systemic beta-adrenergic adverse effects were observed with all active doses and were generally dose-related for (R)-albuterol. XOPENEX Inhalation Solution at a dose of 1.25 mg produced a slightly higher rate of systemic beta-adrenergic adverse effects than the 2.5 mg dose of racemic albuterol sulfate inhalation solution.
In a randomized, double-blind, placebo-controlled, cross-over study, 12 adults with mildto-moderate asthma were challenged with inhaled methacholine chloride 20 and 180 minutes following administration of a single dose of 2.5 mg of racemic albuterol sulfate, 1.25 mg of XOPENEX, 1.25 mg of (S)-albuterol, or placebo using a Pari LC Jet™ nebulizer. Racemic albuterol sulfate, XOPENEX, and (S)-albuterol had a protective effect against methacholine-induced bronchoconstriction 20 minutes after administration, although the effect of (S)-albuterol was minimal. At 180 minutes after administration, the bronchoprotective effect of 1.25 mg of XOPENEX was comparable to that of 2.5 mg of racemic albuterol sulfate. At 180 minutes after administration, 1.25 mg of (S)-albuterol had no bronchoprotective effect.
In a clinical study in adults with mild-to-moderate asthma, comparable efficacy (as measured by change from baseline FEV 1) and safety (as measured by heart rate, blood pressure, ECG, serum potassium, and tremor) were demonstrated after a cumulative dose of 5 mg of XOPENEX Inhalation Solution (four consecutive doses of 1.25 mg administered every 30 minutes) and 10 mg of racemic albuterol sulfate inhalation solution (four consecutive doses of 2.5 mg administered every 30 minutes).
12.3 Pharmacokinetics
Adults and Adolescents ≥12 Years Old
The inhalation pharmacokinetics of XOPENEX Inhalation Solution were investigated in a randomized cross-over study in 30 healthy adults following administration of a single dose of 1.25 mg and a cumulative dose of 5 mg of XOPENEX Inhalation Solution and a single dose of 2.5 mg and a cumulative dose of 10 mg of racemic albuterol sulfate inhalation solution by nebulization using a PARI LC Jet™ nebulizer with a Dura-Neb ® 2000 compressor.
Following administration of a single 1.25 mg dose of XOPENEX Inhalation Solution, exposure to (R)-albuterol (AUC of 3.3 nghr/mL) was approximately 2-fold higher than following administration of a single 2.5 mg dose of racemic albuterol inhalation solution (AUC of 1.7 nghr/mL) (see Table 5). Following administration of a cumulative 5 mg dose of XOPENEX Inhalation Solution (1.25 mg given every 30 minutes for a total of four doses) or a cumulative 10 mg dose of racemic albuterol inhalation solution (2.5 mg given every 30 minutes for a total of four doses), C max and AUC of (R)-albuterol were comparable (see Table 6).
Table 6: Mean (SD) Values for Pharmacokinetic Parameters in Healthy Adults γ Median (Min, Max) reported for T max.
* A negative T max indicates C max occurred between first and last nebulizations.
** Values reflect only (R)-albuterol and do not include (S)-albuterol.
Single Dose Cumulative Dose Racemic Racemic XOPENEX albuterol XOPENEX albuterol 1.25 mg sulfate 2.5 mg 5 mg sulfate 10 mg C max (ng/mL)
(R)-albuterol1.1 (0.45) 0.8 (0.41)** 4.5 (2.20) 4.2 (1.51)** Tmax (h)γ
(R)-albuterol0.2 0.2 0.2 0.2 (0.17, 0.37) (0.17, 1.50) (–0.18*, 1.25) (–0.28*, 1.00) AUC (nghr/mL)
(R)-albuterol3.3 (1.58) 1.7 (0.99)** 17.4 (8.56) 16.0 (7.12)** T ½ (h)
(R)-albuterol3.3 (2.48) 1.5 (0.61) 4.0 (1.05) 4.1 (0.97) Children 6-11 Years Old
The pharmacokinetic parameters of (R)- and (S)-albuterol in children with asthma were obtained using population pharmacokinetic analysis. These data are presented in Table 7. For comparison, adult data obtained by conventional pharmacokinetic analysis from a different study also are presented in Table 7.
In children, AUC and C max of (R)-albuterol following administration of 0.63 mg XOPENEX Inhalation Solution were comparable to those following administration of 1.25 mg racemic albuterol sulfate inhalation solution.
When the same dose of 0.63 mg of XOPENEX Inhalation Solution was given to children and adults, the predicted C max of (R)-albuterol in children was similar to that in adults (0.52 vs. 0.56 ng/mL), while predicted AUC in children (2.55 nghr/mL) was about 1.5-fold higher than that in adults (1.65 nghr/mL). These data support lower doses for children 6-11 years old compared with the adult doses [see Dosage and Administration ( 2) ].
Table 7: (R)-Albuterol Exposure in Adults and Pediatric Subjects (6-11 years) a The values are predicted by assuming linear pharmacokinetics
b The data obtained from Table 6
c Area under the plasma concentration curve from time 0 to infinity
d Maximum plasma concentration
Children 6-11 years Adults ≥12 years Racemic Racemic XOPENEX XOPENEX albuterol albuterol XOPENEX XOPENEX Treatment 0.31 mg 0.63 mg 1.25 mg 2.5 mg 0.63 mg 1.25 mg AUC0-∞
(nghr/mL) c1.36 2.55 2.65 5.02 1.65 a 3.3 b Cmax
(ng/mL) d0.303 0.521 0.553 1.08 0.56 a 1.1 b Metabolism and Elimination
Information available in the published literature suggests that the primary enzyme responsible for the metabolism of albuterol enantiomers in humans is SULT1A3 (sulfotransferase). When racemic albuterol was administered either intravenously or via inhalation after oral charcoal administration, there was a 3- to 4-fold difference in the area under the concentration-time curves between the (R)- and (S)-albuterol enantiomers, with (S)-albuterol concentrations being consistently higher. However, without charcoal pretreatment, after either oral or inhalation administration the differences were 8- to 24-fold, suggesting that (R)-albuterol is preferentially metabolized in the gastrointestinal tract, presumably by SULT1A3.
The primary route of elimination of albuterol enantiomers is through renal excretion (80% to 100%) of either the parent compound or the primary metabolite. Less than 20% of the drug is detected in the feces. Following intravenous administration of racemic albuterol, between 25% and 46% of the (R)-albuterol fraction of the dose was excreted as unchanged (R)-albuterol in the urine.
Special Populations
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of XOPENEX Inhalation Solution has not been evaluated.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of racemic albuterol was evaluated in 5 subjects with creatinine clearance of 7 to 53 mL/min, and the results were compared with those from healthy volunteers. Renal disease had no effect on the half-life, but there was a 67% decline in racemic albuterol clearance. Caution should be used when administering high doses of XOPENEX Inhalation Solution to patients with renal impairment [see Use in Specific Populations ( 8.6) ].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Although there have been no carcinogenesis studies with levalbuterol HCl, racemic albuterol sulfate has been evaluated for its carcinogenic potential.
In a 2-year study in Sprague-Dawley rats, dietary administration of racemic albuterol sulfate resulted in a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at doses of 2 mg/kg/day and greater (approximately 4 times the MRDI dose of levalbuterol HCl for adults and approximately 5 times the MRDI dose of levalbuterol HCl for children on a mg/m 2 basis). In an 18-month study in CD-1 mice and a 22-month study in the golden hamster, dietary administration of racemic albuterol sulfate showed no evidence of tumorigenicity. Dietary doses in CD-1 mice were up to 500 mg/kg/day (approximately 540 times the MRDI dose of levalbuterol HCl for adults and approximately 630 times the MRDI dose of levalbuterol HCl for children on a mg/m 2 basis) and doses in the golden hamster study were up to 50 mg/kg/day (approximately 90 times the MRDI dose of levalbuterol HCl for adults on a mg/m 2 basis and approximately 105 times the MRDI dose of levalbuterol HCl for children on a mg/m 2 basis).
Levalbuterol HCl was not mutagenic in the Ames test or the CHO/HPRT Mammalian Forward Gene Mutation Assay. Levalbuterol HCl was not clastogenic in the in vivo micronucleus test in mouse bone marrow. Racemic albuterol sulfate was not clastogenic in an in vitro chromosomal aberration assay in CHO cell cultures.
No fertility studies have been conducted with levalbuterol hydrochloride. Reproduction studies in rats using racemic albuterol sulfate demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg/day (approximately 108 times the maximum recommended daily inhalation dose of levalbuterol HCl for adults on a mg/m 2 basis).
-
14 CLINICAL STUDIES
Adults and Adolescents ≥12 Years Old
The safety and efficacy of XOPENEX Inhalation Solution were evaluated in a 4-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study in 362 adult and adolescent patients 12 years of age and older, with mild-to-moderate asthma (mean baseline FEV 1 60% of predicted). Approximately half of the patients were also receiving inhaled corticosteroids. Patients were randomized to receive XOPENEX 0.63 mg, XOPENEX 1.25 mg, racemic albuterol sulfate 1.25 mg, racemic albuterol sulfate 2.5 mg, or placebo three times a day administered via a PARI LC Plus™ nebulizer and a Dura-Neb ® portable compressor. Racemic albuterol delivered by a chlorofluorocarbon (CFC) metered-dose inhaler (MDI) was used on an as-needed basis as the rescue medication.
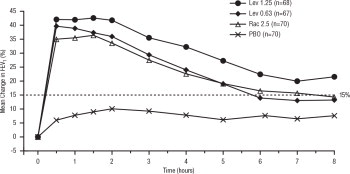
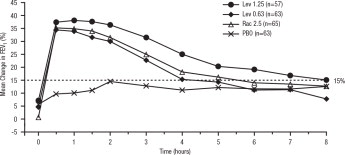
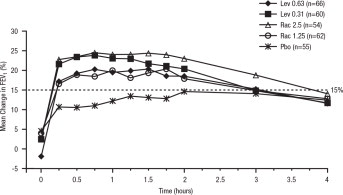
Efficacy, as measured by the mean percent change from baseline FEV 1, was demonstrated for all active treatment regimens compared with placebo on day 1 and day 29. On both day 1 (see Figure 1) and day 29 (see Figure 2), 1.25 mg of XOPENEX demonstrated the largest mean percent change from baseline FEV 1 compared with the other active treatments. A dose of 0.63 mg of XOPENEX and 2.5 mg of racemic albuterol sulfate produced a clinically comparable mean percent change from baseline FEV 1 on both day 1 and day 29.
Figure 1: Mean Percent Change from Baseline FEV1 on Day 1, Adults and Adolescents ≥12 years old
Figure 2: Mean Percent Change from Baseline FEV1 on Day 29, Adults and Adolescents ≥12 years old
The mean time to onset of a 15% increase in FEV 1 over baseline for levalbuterol at doses of 0.63 mg and 1.25 mg was approximately 17 minutes and 10 minutes, respectively, and the mean time to peak effect for both doses was approximately 1.5 hours after 4 weeks of treatment. The mean duration of effect, as measured by a >15% increase from baseline FEV 1, was approximately 5 hours after administration of 0.63 mg of levalbuterol and approximately 6 hours after administration of 1.25 mg of levalbuterol after 4 weeks of treatment. In some patients, the duration of effect was as long as 8 hours.
Children 6-11 Years Old
A multicenter, randomized, double-blind, placebo- and active-controlled study was conducted in children with mild-to-moderate asthma (mean baseline FEV 1 73% of predicted) (n=316). Following a 1-week placebo run-in, subjects were randomized to XOPENEX (0.31 or 0.63 mg), racemic albuterol (1.25 or 2.5 mg), or placebo, which were delivered three times a day for 3 weeks using a PARI LC Plus™ nebulizer and a Dura-Neb ® 3000 compressor.
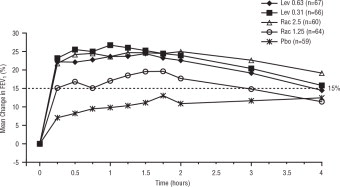
Efficacy, as measured by mean peak percent change from baseline FEV 1, was demonstrated for all active treatment regimens compared with placebo on day 1 and day 21. Time profile FEV 1 curves for day 1 and day 21 are shown in Figure 3 and Figure 4, respectively. The onset of effect (time to a 15% increase in FEV 1 over test-day baseline) and duration of effect (maintenance of a >15% increase in FEV 1 over test-day baseline) of levalbuterol were clinically comparable to those of racemic albuterol.
Figure 3: Mean Percent Change from Baseline FEV1 on Day 1, Children 6-11 Years of Age
Figure 4: Mean Percent Change from Baseline FEV1 on Day 21, Children 6-11 Years of Age
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XOPENEX Inhalation Solution is supplied in 3 mL unit-dose, low-density polyethylene (LDPE) vials as a clear, colorless, sterile, preservative-free, aqueous solution, in three different strengths of levalbuterol (0.31 mg, 0.63 mg, 1.25 mg). Each strength of XOPENEX Inhalation Solution is available in a shelf-carton containing one or more foil pouches, each containing 12 unit-dose LDPE vials.
XOPENEX (levalbuterol HCl) Inhalation Solution, 0.31 mg (foil pouch label color green) contains 0.31 mg of levalbuterol (as 0.36 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC: 17478-172-24).
XOPENEX (levalbuterol HCl) Inhalation Solution, 0.63 mg (foil pouch label color yellow) contains 0.63 mg of levalbuterol (as 0.73 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC: 17478-173-24).
XOPENEX (levalbuterol HCl) Inhalation Solution, 1.25 mg (foil pouch label color red) contains 1.25 mg of levalbuterol (as 1.44 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC: 17478-174-24).
XOPENEX Inhalation Solution is also available as a concentrate in individually pouched 0.5 mL unit-dose vials containing 1.25 mg of levalbuterol (NDC: 17478-171-30).
Store XOPENEX Inhalation Solution in the protective foil pouch at 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature]. Protect from light and excessive heat. Keep unopened vials in the foil pouch. Once the foil pouch is opened, the vials should be used within 2 weeks. Vials removed from the pouch, if not used immediately, should be protected from light and used within 1 week. Discard any vial if the solution is not colorless.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling ( Patient Information and Instructions for Using XOPENEX Inhalation Solution).
Patients should be given the following information:
Hypersensitivity
Query patients about previously experienced hypersensitivity to levalbuterol or racemic albuterol and counsel patients to report any hypersensitivity reactions to their physician.
Frequency of Use
Inform patients not to increase the dose or use XOPENEX Inhalation Solution more frequently than recommended without consulting their physician. If patients find that treatment with XOPENEX Inhalation Solution becomes less effective for symptomatic relief, symptoms become worse, or they need to use the product more frequently than usual, they should seek medical attention immediately.
Paradoxical Bronchospasm
Inform patients that XOPENEX Inhalation Solution can produce paradoxical bronchospasm. Instruct patients to discontinue XOPENEX Inhalation Solution if paradoxical bronchospasm occurs.
Concomitant Drug Use
Inform patients using XOPENEX Inhalation Solution, that other inhaled drugs and asthma medications should be taken only as directed by their physician.
Common Adverse Reactions
Advise patients of the common adverse reactions of treatment with XOPENEX Inhalation Solution including palpitations, chest pain, fast heart rate, headache, dizziness, tremor and nervousness.
Pregnancy
Advise patients who are pregnant or nursing to contact their physician about the use of XOPENEX Inhalation Solution.
General Information on Storage and Use
Advise patients to store XOPENEX Inhalation Solution in the foil pouch between 20°C to 25°C (68°F to 77°F) protected from light and excessive heat. Do not use after the expiration date stamped on the container. Store unused vials in the protective foil pouch. Once the foil pouch is opened, use the vials within 2 weeks. Use vials removed from the pouch, immediately, or protect from light and use within 1 week. Discard any vial if the solution is not colorless.
Advise patients not to mix XOPENEX Inhalation Solution with other drugs in a nebulizer.
AKORN
Distributed by: Akorn, Inc.
Lake Forest, IL 60045Manufactured for: Oak Pharmaceuticals, Inc.
For customer service, call 1-800-932-5676.
To report adverse events, call 1-800-932-5676.
For medical information, call 1-800-932-5676.Xopenex is a registered trademark of Sunovion Pharmaceuticals Inc. and is used under license.
XP00N June 2017
AKNX024-642R01 -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
XOPENEX®(pronounced zō-pen-eks)
(levalbuterol hydrochloride)
Inhalation Solution
0.31 mg, 0.63 mg, 1.25 mg
3 mL Unit-Dose Vials
For Oral Inhalation Only
XOPENEX Inhalation Solution is only for use with a nebulizer.Read this Patient Information before you start to use XOPENEX Inhalation Solution and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is XOPENEX Inhalation Solution?
XOPENEX Inhalation Solution is an inhaled prescription medicine used for the treatment or prevention of bronchospasm in people 6 years of age and older.
XOPENEX Inhalation Solution has not been shown to be safe and effective in children younger than 6 years of age.
XOPENEX Inhalation Solution is supplied in 3 mL unit-dose vials in three different strengths of levalbulterol (0.31 mg, 0.63 mg, 1.25 mg). The vials do not require dilution before use.
Who should not use XOPENEX Inhalation Solution?
Do not use XOPENEX Inhalation Solution if you are allergic to levalbuterol, racemic albuterol, or any of the ingredients in XOPENEX. See the end of this leaflet for a complete list of ingredients in XOPENEX Inhalation Solution.
What should I tell my doctor before using XOPENEX Inhalation Solution?
Before you use XOPENEX Inhalation Solution, tell your doctor if you have:
- had an allergic reaction to levalbuterol or racemic albuterol
- heart problems
- high blood pressure
- seizures
- diabetes
- thyroid problems
- any other medical conditions
- are pregnant or planning to become pregnant. It is not known if XOPENEX Inhalation Solution will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if XOPENEX Inhalation Solution passes into your breast milk. You and your doctor should decide if you will use XOPENEX Inhalation Solution or breastfeed. You should not do both.
Tell your doctor about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. XOPENEX Inhalation Solution may affect the way other medicines work, and other medicines may affect how XOPENEX Inhalation Solution works.
Especially tell your doctor if you take:
- other asthma medicines
- heart medicines
- medicines that increase urination (diuretics)
- antidepressants
- medicine to treat chronic obstructive pulmonary disease (COPD)
Ask your doctor if you are not sure if any of your medicines are the kinds listed above.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I use XOPENEX Inhalation Solution?
- Read the step-by-step Instructions for Using XOPENEX Inhalation Solution at the end of this leaflet.
- Use XOPENEX Inhalation Solution exactly as your doctor tells you to. Do not change your dose without talking to your doctor first.
- Your doctor will tell you how many times and when to use your XOPENEX Inhalation Solution.
- An adult should help a child use XOPENEX Inhalation Solution.
- Do not use your XOPENEX Inhalation Solution more often than your doctor tells you to.
-
Get medical help right away if XOPENEX Inhalation Solution:
- does not work as well for your asthma symptoms or
- your asthma symptoms get worse or
- you need to use your XOPENEX Inhalation Solution more often than usual
- If you also use another medicine by inhalation, you should ask your doctor for instructions on when to use it while you are also using XOPENEX Inhalation Solution.
- Do not mix XOPENEX Inhalation Solution with other medicines in your nebulizer.
- Only use XOPENEX Inhalation Solution if it is colorless. Throw away the XOPENEX Inhalation Solution vial if the liquid medicine is not colorless.
- Do not use XOPENEX Inhalation Solution after the expiration date on the vial.
What are the possible side effects of XOPENEX Inhalation Solution?
XOPENEX Inhalation Solution can cause serious side effects including:
- sudden shortness of breath (bronchospasm). Sudden shortness of breath can happen right away after using XOPENEX Inhalation Solution.
- worsening asthma
- heart problems
- death. If you use too much XOPENEX Inhalation Solution you can have heart or lung problems that can lead to death.
-
serious allergic reactions. Call your doctor and stop using XOPENEX Inhalation Solution right away if you have any symptoms of an allergic reaction such as:
- swelling of the face, throat or tongue
- hives
- rash
- breathing problems
- low potassium levels in your blood
Call your doctor or go to the nearest hospital emergency room right away if you have any of the serious side effects listed above or if you have worsening lung symptoms.
The most common side effects of XOPENEX Inhalation Solution include:
- palpitations
- chest pain
- fast heart rate
- headache
- dizziness
- tremor
- nervousness
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all the possible side effects of XOPENEX Inhalation Solution. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store XOPENEX Inhalation Solution?
- Store unopened XOPENEX Inhalation Solution vials in the protective foil pouch they come in between 68°F to 77°F (20°C to 25°C).
- Keep XOPENEX Inhalation Solution away from light and heat.
- When a XOPENEX Inhalation Solution foil pouch is opened, use the vials within 2 weeks.
- When XOPENEX Inhalation Solution vials are removed from the foil pouch, use them right away or within 1 week.
Keep XOPENEX Inhalation Solution and all medicines out of the reach of children.
General information about the safe and effective use of XOPENEX Inhalation Solution.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use XOPENEX Inhalation Solution for a condition for which it was not prescribed. Do not give XOPENEX Inhalation Solution to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about XOPENEX Inhalation Solution. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about XOPENEX Inhalation Solution that is written for health professionals.
For customer service, call 1-800-932-5676.
To report adverse events, call 1-800-932-5676.
For medical information, call 1-800-932-5676.What are the ingredients in XOPENEX Inhalation Solution?
Active ingredient: levalbuterol hydrochloride
Inactive ingredients: sodium chloride, sulfuric acid, water and nitrogen
-
INSTRUCTIONS FOR USE
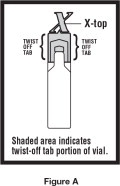
Instructions for Using XOPENEX Inhalation Solution XOPENEX Inhalation Solution vial (see Figure A):
Using your XOPENEX Inhalation Solution:
Read the following Steps before using your XOPENEX Inhalation Solution. If you have any questions, ask your doctor or pharmacist.
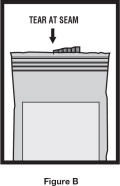
Step 1. Open the foil pouch by tearing the notched edge along the seam of the pouch ( See Figure B). Remove 1 vial to be used right away. Keep the rest of the unused vials in the foil pouch to protect them from light and heat.
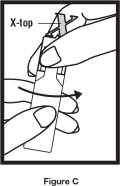
Step 2. Hold the vial in your hands. Make sure your thumb and finger cover the twist-off tabs below the X-top ( See Figure C).
Step 3. While holding the top firmly between your thumb and finger, twist the body of the vial to open the vial ( See Figure C).
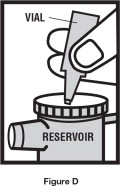
Step 4. Throw away the top of the vial and squeeze the entire contents of the vial into the nebulizer reservoir ( See Figure D).
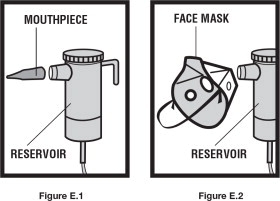
Step 5. Connect the nebulizer reservoir to the mouthpiece ( See Figure E.1) or face mask ( See Figure E.2).
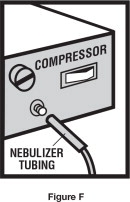
Step 6. Connect the nebulizer to the compressor ( See Figure F).
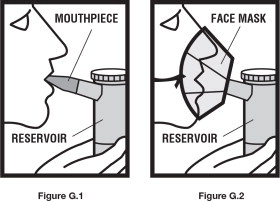
Step 7. Sit in a comfortable, upright position. Place the mouthpiece in your mouth ( See Figure G.1) or put on your face mask ( See Figure G.2). Turn on the compressor.
Step 8. Breathe as calmly, deeply, and evenly as possible until no more mist is seen in the nebulizer reservoir. Your treatment will take about 5 to 15 minutes. When you do not see any mist in the nebulizer reservoir, your treatment is finished.
Step 9. Clean and store your nebulizer. See the manufacturer's instructions that come with your nebulizer for how to clean and store your nebulizer.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
AKORN
Distributed by: Akorn, Inc.
Lake Forest, IL 60045Manufactured for: Oak Pharmaceuticals, Inc.
For customer service, call 1-800-932-5676.
To report adverse events, call 1-800-932-5676.
For medical information, call 1-800-932-5676.Xopenex is a registered trademark of Sunovion Pharmaceuticals Inc. and is used under license.
XP00N June 2017
AKNX024-642R01 -
PRINCIPAL DISPLAY PANEL
DRUG: Xopenex
GENERIC: Levalbuterol Hydrochloride
DOSAGE: SOLUTION
ADMINSTRATION: RESPIRATORY (INHALATION)
NDC: 70518-1227-0
PACKAGING: 3 mL in 1 VIAL, SINGLE-DOSE
OUTER PACKAGING: 12 in 1 POUCH
ACTIVE INGREDIENT(S):
- Levalbuterol Hydrochloride 1.25mg in 3mL
INACTIVE INGREDIENT(S):
- Nitrogen
- Water
- Sodium Chloride
- Sulfuric Acid

-
INGREDIENTS AND APPEARANCE
XOPENEX
levalbuterol hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70518-1227(NDC:17478-174) Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVALBUTEROL HYDROCHLORIDE (UNII: WDQ1526QJM) (LEVALBUTEROL - UNII:EDN2NBH5SS) LEVALBUTEROL 1.25 mg in 3 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SULFURIC ACID (UNII: O40UQP6WCF) WATER (UNII: 059QF0KO0R) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70518-1227-0 2 in 1 CARTON 05/25/2018 1 12 in 1 POUCH 1 3 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020837 05/25/2018 Labeler - REMEDYREPACK INC. (829572556)
Trademark Results [Xopenex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XOPENEX 77138209 3655211 Live/Registered |
SUNOVION PHARMACEUTICALS INC. 2007-03-22 |
 XOPENEX 77138201 3651698 Live/Registered |
SUNOVION PHARMACEUTICALS INC. 2007-03-22 |
 XOPENEX 75468045 2350721 Live/Registered |
SUNOVION PHARMACEUTICALS INC. 1998-04-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.