EPIOXA CROSS-LINKING- riboflavin 5-phosphate ophthalmic kit
EPIOXA CROSS-LINKING by
Drug Labeling and Warnings
EPIOXA CROSS-LINKING by is a Prescription medication manufactured, distributed, or labeled by Glaukos Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EPIOXA™ HD and EPIOXA™ safely and effectively. See full prescribing information for EPIOXA HD and EPIOXA.
EPIOXA HD (riboflavin 5'-phosphate ophthalmic solution) 0.239%, for topical ophthalmic use
EPIOXA (riboflavin 5'-phosphate ophthalmic solution) 0.177%, for topical ophthalmic use
For use with the O2n™ System and Boost Goggles®
Initial U.S. Approval: 2016INDICATIONS AND USAGE
EPIOXA HD and EPIOXA are photoenhancers indicated for use in epithelium-on corneal collagen cross-linking for the treatment of keratoconus in adults and pediatric patients aged 13 years and older, in conjunction with the O2n System and the Boost Goggles (1).
DOSAGE AND ADMINISTRATION
- Apply topical anesthetic and insert a lid speculum (2).
- Using a cellulose spear sponge soaked with EPIOXA HD, remove the mucin layer from the corneal surface without debriding the corneal epithelium (epithelium-on) by swiping the sponge 4 to 10 times horizontally and vertically (2).
- Apply two drops of EPIOXA HD topically on the eye every 60 seconds for 4 minutes (STEP 1), followed by two drops of EPIOXA topically on the eye every 30 seconds for 6 minutes (STEP 2) (2).
- Gently rinse the corneal surface with approximately 5 mL of balanced salt solution (BSS) (2).
- Perform ultrasound pachymetry. If corneal thickness is less than 325 microns, irradiation should not be performed (2).
- Apply the Boost Goggles and turn on the oxygen flow. Refer to the Boost Goggles User Guide (2).
- Center the optical head of the O2n System over the cornea and irradiate the eye as per the instructions in the O2n System Operator's Manual. The O2n System automatically delivers irradiation to the eye for 11 minutes 6 seconds at 30 mW/cm2 with an on/off cycle of 1 second UV-A on/ 1 second UV-A off at a wavelength of 365 nm (2).
- Instill BSS on the cornea every 2 minutes, or more frequently as needed, to maintain corneal hydration during UV-A irradiation (2).
- When the UV-A irradiation has stopped, shut off the oxygen flow and remove the Boost Goggles and lid speculum (2).
- Apply a bandage contact lens (2).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Herpetic keratitis: Use with caution in patients with a history of herpetic keratitis due to the potential for reactivation (5).
ADVERSE REACTIONS
The most common adverse reaction was conjunctival hyperaemia (31%). Other adverse reactions, occurring in 5% to 25% of eyes included: corneal opacity (haze), photophobia, punctate keratitis, eye pain, eye irritation, increased lacrimation, corneal epithelium defect, eyelid oedema, corneal striae, visual acuity reduced, dry eye, and anterior chamber flare (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact GLAUKOS CORPORATION at 1-888-404-1644 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
3.1 EPIOXA HD
3.2 EPIOXA
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Aphakic and Pseudophakic Patients
5 WARNINGS AND PRECAUTIONS
5.1 Herpetic Keratitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
EPIOXA HD and EPIOXA are for topical ophthalmic use. NOT for injection or intraocular use.
EPIOXA HD and EPIOXA are supplied in single-dose syringes. Discard opened syringes after use.
EPIOXA HD and EPIOXA are for use with the O2n System and Boost Goggles only.
Refer to the O2n System Operator's Manual and Boost Goggles User Guide for device instructions.
2.2 Recommended Dosage and Administration Instructions
Apply topical anesthetic and insert a lid speculum.
Using a cellulose spear sponge soaked with EPIOXA HD, remove the mucin layer from the corneal surface without debriding the corneal epithelium (epithelium-on) by swiping the sponge 4 to 10 times horizontally and vertically.
Apply two drops of EPIOXA HD topically on the eye every 60 seconds for 4 minutes (STEP 1), followed by two drops of EPIOXA topically on the eye every 30 seconds for 6 minutes (STEP 2).
Gently rinse the corneal surface with approximately 5 mL of balanced salt solution (BSS).
Perform ultrasound pachymetry. If corneal thickness is less than 325 microns, irradiation should not be performed.
Apply the Boost Goggles and turn on the oxygen flow as per the instructions in the Boost Goggles User Guide.
Center the optical head of the O2n System over the cornea and irradiate the eye as per the instructions in the O2n System Operator's Manual. The O2n System automatically delivers irradiation to the eye for 11 minutes 6 seconds at 30 mW/cm2 with an on/off cycle of 1 second UV-A on/1 second UV-A off at a wavelength of 365 nm.
Instill BSS on the cornea every 2 minutes, or more frequently as needed, to maintain corneal hydration during UV-A irradiation.
When the UV-A irradiation has stopped, shut off the oxygen flow, and remove the Boost Goggles and lid speculum.
Apply a bandage contact lens.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
Herpetic keratitis [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of EPIOXA HD and EPIOXA in the epithelium-on corneal collagen cross-linking procedure with UV-A irradiation and supplemental oxygen was evaluated in two randomized, parallel-group, sham procedure/vehicle-controlled trials. Study eyes were randomized in a 2:1 treatment allocation to receive corneal collagen cross-linking (CXL) or sham procedure/vehicle control at the baseline visit. In both trials, CXL-treated eyes were followed for 12 months.
Safety data were obtained from a total of 389 CXL-treated eyes.
The most commonly reported adverse reaction in CXL-treated eyes was conjunctival hyperaemia (31%). Other adverse reactions occurring in 5% to 25% of CXL-treated eyes included: corneal opacity (haze), photophobia, punctate keratitis, eye pain, eye irritation, lacrimation increased, corneal epithelium defect, eyelid oedema, corneal striae, visual acuity reduced, dry eye, and anterior chamber flare.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Animal development and reproduction studies have not been conducted with EPIOXA HD and EPIOXA with the O2n System and Boost Goggles. Since it is not known whether the epithelium-on corneal collagen cross-linking procedure can cause fetal harm or affect reproduction capacity, it should not be performed on pregnant women.
8.2 Lactation
Risk Summary
There are no data on the presence of EPIOXA HD or EPIOXA in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother's clinical need for the EPIOXA HD and EPIOXA epithelium-on corneal collagen cross-linking procedure and any potential adverse effects on the breastfed child from the EPIOXA HD and EPIOXA epithelium-on corneal collagen cross-linking procedure or from the underlying maternal condition.
-
11 DESCRIPTION
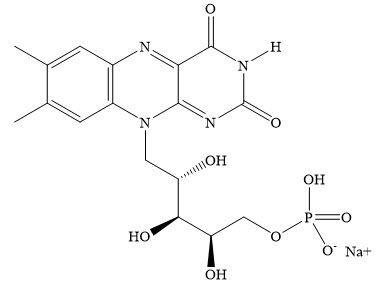
EPIOXA HD (riboflavin 5'-phosphate ophthalmic solution) 0.239% and EPIOXA (riboflavin 5'-phosphate ophthalmic solution) 0.177% contain riboflavin 5'-phosphate, a photoenhancer, for topical ophthalmic use.
EPIOXA HD 0.239% is a clear, yellow, sterile buffered solution containing 2.39 mg/mL riboflavin 5'-phosphate. The pH of the solution is 6.7 to 7.7 and the osmolality is 200 mOsm/kg to 260 mOsm/kg. Each mL of solution contains 2.50 mg of riboflavin 5'-phosphate sodium (equivalent to 1.97 mg/mL riboflavin). Riboflavin 5'-phosphate sodium is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are hydroxypropyl methylcellulose, sodium chloride, dibasic sodium phosphate dihydrate, edetate disodium dihydrate, tromethamine, monobasic sodium phosphate dihydrate, benzalkonium chloride, and water for injection. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH.
EPIOXA 0.177% is a clear, yellow, sterile buffered solution containing 1.77 mg/mL riboflavin 5'-phosphate. The pH of the solution is 6.5 to 7.5 and the osmolality is 330 mOsm/kg to 400 mOsm/kg. Each mL of solution contains 1.85 mg of riboflavin 5'-phosphate sodium (equivalent to 1.46 mg/mL riboflavin). Riboflavin 5'-phosphate sodium is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are sodium chloride, dibasic sodium phosphate dihydrate, tromethamine, monobasic sodium phosphate dihydrate, and water for injection. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH.
The molecular formula for riboflavin 5'-phosphate sodium (Vitamin B2) is C17H20N4NaO9P with a molecular weight of 478.33 g/mol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Riboflavin 5'-phosphate sodium (Vitamin B2) is the precursor of two coenzymes, flavin adenine dinucleotide and flavin mononucleotide, which catalyze oxidation/reduction reactions involved in a number of metabolic pathways.
Under the conditions used for corneal collagen cross-linking, riboflavin 5'-phosphate functions as a photoenhancer and generates singlet oxygen which is responsible for the cross-linking.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Animal studies have not been conducted to determine the carcinogenic potential of photoexcited riboflavin.
Mutagenesis
Photoexcited riboflavin has been shown to be genotoxic in the Ames Salmonella reverse mutation assay and in the SOS/umu test system.
The genotoxicity of riboflavin, in the absence of photoexcitation has been examined in vitro in bacterial reverse mutation assays, sister chromatid exchange assay, chromosomal aberration assays and in vivo in a mouse micronucleus study. The overall weight of evidence indicates that riboflavin, in the absence of photoexcitation, is not genotoxic.
Impairment of Fertility
Animal studies to determine the effects of the EPIOXA HD and EPIOXA epithelium-on corneal collagen cross-linking procedure on fertility were not conducted.
-
14 CLINICAL STUDIES
Two prospective, randomized, parallel-group, sham procedure/vehicle-controlled trials (Study 1 [NCT03442751] and Study 2 [NCT05759559]) were conducted to evaluate the safety and efficacy of epithelium-on corneal collagen cross-linking (CXL) using riboflavin 5'-phosphate ophthalmic solutions with UV-A irradiation and supplemental oxygen in patients with keratoconus.
In both trials, eligible eyes were randomized to receive CXL treatment or sham procedure/vehicle control in a 2:1 treatment allocation at the baseline visit. Aphakic patients and pseudophakic patients without a UV-blocking intraocular lens were excluded. Both eyes of a patient could be enrolled in the trial; however, one eye was treated first, and the second eye was treated between 1 week and 3 months after the first eye. Eyes were evaluated at 1 day, 3 days, 1 week, and 1, 3, 6, and 12 months post-treatment.
In Study 1, eyes randomized to sham procedure/vehicle control were permitted to receive CXL treatment after month 6 and were followed an additional 6 months. The primary efficacy endpoint was at month 6 post-treatment and the secondary efficacy endpoint was at month 12 post-treatment. In Study 2, the primary efficacy endpoint was at month 12 post-treatment and the secondary efficacy endpoint was at month 6 post-treatment.
In Study 1, a total of 280 eyes of 201 patients were randomized into the trial, of which 279 eyes were treated: 189 eyes received CXL treatment and 90 eyes initially received sham procedure/vehicle control. A statistically significant treatment effect was demonstrated at month 6, based on the difference in change from baseline in maximum corneal curvature (Kmax) between the CXL treatment group and sham procedure/vehicle control group (Table 1).
Table 1. Study 1: Mean Baseline Kmax (D) and Change from Baseline Kmax (D) Visit CXL Treatment
(N=189)Sham Procedure/ Vehicle Control
(N=90)Treatment Difference
(95% CI) P-valueRandomized eyes that received study treatment - * Mean (standard deviation) Kmax
- † LS Mean change from baseline and corresponding 95% CIs obtained from a RMMM ANCOVA model with treatment as a factor and the baseline Kmax value and baseline keratoconus severity as covariates. Missing post-baseline Kmax data were handled by multiple imputation procedure for month 6.
- ‡ Missing post-baseline Kmax data were handled by last observation carried forward method for month 12 in Sham Procedure/Vehicle Control eyes that received CXL treatment after month 6.
Baseline* 59.4 (9.1) 59.3 (9.1) Month 6† -0.3 (-0.6, -0.0) 0.6 (0.2, 1.1) -1.0 (-1.5, -0.4) P<0.01 Month 12‡ -0.4 (-0.7, -0.2) 0.7 (0.3, 1.1) -1.1 (-1.6, -0.6) P<0.01 In a subgroup analysis of patients in this trial at month 6, younger patients (< 29 years) experienced a treatment effect of -2.0 D, as a combination of improvement in the CXL treatment arm (-0.7 D) and deterioration in the sham procedure/vehicle control arm (1.3 D). Older patients (≥ 29 years) did not experience improvement at month 6 in either arm.
In Study 2, a total of 312 eyes of 208 patients were randomized into the trial, of which 312 eyes were treated: 200 eyes received CXL treatment and 112 eyes received sham procedure/vehicle control. A statistically significant treatment effect was demonstrated at month 12, based on the difference in change from baseline in Kmax between the CXL treatment group and sham procedure/vehicle control group (Table 2).
Table 2. Study 2: Mean Baseline Kmax (D) and Change from Baseline Kmax (D) Visit CXL Treatment
(N=200)Sham Procedure/ Vehicle Control
(N=112)Treatment Difference
(95% CI) P-ValueRandomized eyes that received study treatment - * Mean (standard deviation) Kmax
- † LS Mean change from baseline and corresponding 95% CIs obtained from a RMMM ANCOVA model with treatment as a factor and the baseline Kmax value and baseline keratoconus severity and age stratum as covariates. Missing post-baseline Kmax data were handled by multiple imputation procedure.
Baseline* 58.0 (8.0) 58.1 (8.6) Month 6† -0.4 (-0.6, -0.2) 0.1 (-0.1, 0.4) -0.6 (-0.9, -0.2) P<0.01 Month 12† -0.5 (-0.7, -0.3) 0.4 (0.1, 0.8) -1.0 (-1.3, -0.6) P<0.01 In a subgroup analysis of patients in this trial at month 12, younger patients (< 30 years) experienced a treatment effect of -1.1 D, as a combination of improvement in the CXL treatment arm (-0.5 D) and deterioration in the sham procedure/vehicle control arm (0.5 D). Older patients (≥ 30 years) in the CXL treatment arm experienced comparable improvement (-0.6 D) as younger patients (-0.5 D); however, in the sham procedure/vehicle control arm, older patients (-0.0 D) did not deteriorate as much as younger patients (0.5 D).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
EPIOXA HD (riboflavin 5'-phosphate ophthalmic solution) 0.239%, and EPIOXA (riboflavin 5'-phosphate ophthalmic solution) 0.177%, are clear, yellow, ophthalmic solutions. EPIOXA HD and EPIOXA are co-packaged in an Epithelium-on Cross-linking Kit (NDC: 25357-024-01) containing:
- One single-dose glass syringe containing 2 mL of EPIOXA HD 0.239% packaged in a foil pouch.
- One single-dose glass syringe containing 2 mL of EPIOXA 0.177% packaged in a foil pouch.
Store kit refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Minimize exposure of the syringes to light once removed from their protective packaging.
For topical ophthalmic use. Single-dose only. Discard syringes after use.
EPIOXA HD and EPIOXA are for use only with the O2n System, the single-use O2n System Treatment Activation Card, and Boost Goggles.
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 25357-024-01
Rx Only
Epithelium-on Cross-linking Kit
Epioxa™ HD
(riboflavin 5'- phosphate ophthalmic solution) 0.239%co-packaged with
Epioxa™
(riboflavin 5'- phosphate ophthalmic solution) 0.177%For Topical Ophthalmic Use Only
Use only with O2n™ System, O2n™ System Single-use Treatment
Activation Card, and Boost Goggles®.
Do NOT use if damaged. Single-dose. Discard unused portion.GLAUKOS®

-
INGREDIENTS AND APPEARANCE
EPIOXA CROSS-LINKING
riboflavin 5-phosphate ophthalmic kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25357-024 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25357-024-01 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 01/16/2026 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 2 mL Part 2 1 SYRINGE, GLASS 2 mL Part 1 of 2 EPIOXA HD
riboflavin 5-phosphate solution/ dropsProduct Information Item Code (Source) NDC: 25357-026 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIBOFLAVIN 5'-PHOSPHATE (UNII: 7N464URE7E) (RIBOFLAVIN 5'-PHOSPHATE - UNII:7N464URE7E) RIBOFLAVIN 5'-PHOSPHATE 2.39 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25357-026-01 1 in 1 POUCH 1 2 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219910 01/16/2026 Part 2 of 2 EPIOXA
riboflavin 5-phosphate solution/ dropsProduct Information Item Code (Source) NDC: 25357-027 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIBOFLAVIN 5'-PHOSPHATE (UNII: 7N464URE7E) (RIBOFLAVIN 5'-PHOSPHATE - UNII:7N464URE7E) RIBOFLAVIN 5'-PHOSPHATE 1.77 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25357-027-01 1 in 1 POUCH 1 2 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219910 01/16/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219910 01/16/2026 Labeler - Glaukos Corporation (012835406)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.