ALTRENO- tretinoin lotion
Altreno by
Drug Labeling and Warnings
Altreno by is a Prescription medication manufactured, distributed, or labeled by Bausch Health US, LLC, Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALTRENO safely and effectively. See full prescribing information for ALTRENO.
ALTRENO™ (tretinoin) lotion, for topical use
Initial U.S. Approval: 1973INDICATIONS AND USAGE
ALTRENO is a retinoid indicated for
The topical treatment of acne vulgaris in patients 9 years of age and older. (1)DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Skin Irritation: Dryness, pain, erythema, irritation and exfoliation may occur with use of ALTRENO. (5.1)
- Ultraviolet Light and Environmental Exposure: Minimize exposure to sunlight and sunlamps. Use sunscreen and protective clothing when sun exposure cannot be avoided. (5.2)
- Fish Allergies: Use ALTRENO with caution if allergic to fish due to potential for allergenicity to fish protein. Advise patients to contact their healthcare provider if they develop pruritus or urticaria. (5.3)
ADVERSE REACTIONS
- The most common adverse reactions occurring in ≥1% of subjects and greater than vehicle were dryness, pain, erythema, irritation and exfoliation (all at the application site). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Skin Irritation
5.2 Ultraviolet Light and Environmental Exposure
5.3 Fish Allergies
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Skin Irritation
Patients using ALTRENO may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of ALTRENO, or discontinue use. Avoid application of ALTRENO to eczematous or sunburned skin.
5.2 Ultraviolet Light and Environmental Exposure
Minimize unprotected exposure to ultraviolet light including sunlight and sunlamps during the use of ALTRENO. Warn patients who normally experience high levels of sun exposure and those with inherent sensitivity to sun to exercise caution. Use sunscreen products and protective clothing over treated areas when sun exposure cannot be avoided.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In 2 randomized, double-blind, vehicle-controlled trials, subjects age 9 years and older applied ALTRENO or vehicle once daily for 12 weeks. The majority of subjects were White (74%) and female (55%). Approximately 47% were Hispanic/Latino and 45% were younger than 18 years of age. Adverse reactions reported by ≥1% of subjects treated with ALTRENO and more frequently than vehicle are summarized in Table 1.
Table 1: Adverse Reactions Reported by ≥1% of Subjects Treated with ALTRENO and More Frequently than Vehicle Adverse Reactions
n (%)ALTRENO
N=767Vehicle
N=783Application site dryness
29 (4)
1 (<1)
Application site pain
25 (3)
3 (<1)
Application site erythema
12 (2)
1 (<1)
Application site irritation
7 (1)
1 (<1)
Application site exfoliation
6 (1)
- 3 (<1)
Application site pain defined as application site stinging, burning or pain.
Skin irritation was evaluated by active assessment of erythema, scaling, hypopigmentation, hyperpigmentation, itching, burning and stinging. The percentage of subjects who were assessed to have these signs and symptoms at any post baseline visit are summarized in Table 2.Table 2: Application Site Tolerability Reactions at Any Post Baseline Visit ALTRENO
N=760
Mild/Mod/Severe
Vehicle
N=782
Mild/Mod/Severe
Erythema
51%
44%
Scaling
49%
30%
Hypopigmentation
12%
10%
Hyperpigmentation
35%
35%
Itching
35%
28%
Burning
30%
14%
Stinging
21%
8%
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published observational studies of topical tretinoin in pregnant women have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are no data on ALTRENO use in pregnant women.
The systemic levels following topical administration are lower than with administration of oral tretinoin; however, absorption of this product may result in fetal exposure. There are reports of major birth defects similar to those seen in infants exposed to oral retinoids, but these case reports do not establish a pattern or association with tretinoin-related embryopathy (see Data).
Animal reproduction studies have not been conducted with ALTRENO. Topical administration of tretinoin in a different formulation to pregnant rats during organogenesis was associated with malformations (craniofacial abnormalities [hydrocephaly], asymmetrical thyroids, variations in ossification, and increased supernumerary ribs) at doses up to 0.5 mg tretinoin/kg/day, approximately 2 times the maximum recommended human dose (MRHD) based on body surface area (BSA) comparison and assuming 100% absorption. Oral administration of tretinoin to pregnant cynomolgus monkeys during organogenesis was associated with malformations at 10 mg/kg/day (approximately 100 times the MRHD based on BSA comparison and assuming 100% absorption) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, and other adverse outcomes. The background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from multiple prospective controlled observational studies on the use of topical tretinoin products during pregnancy have not identified an association with topical tretinoin and major birth defects or miscarriage. The available studies have methodologic limitations, including small sample size and in some cases, lack of physical exam by an expert in birth defects. There are published case reports of infants exposed to topical tretinoin during the first trimester that describe major birth defects similar to those seen in infants exposed to oral retinoids; however, no pattern of malformations has been identified and no causal association has been established in these cases. The significance of these spontaneous reports in terms of risk to the fetus is not known.
Animal Data
Tretinoin in a 0.05% gel formulation was topically administered to pregnant rats during organogenesis at doses of 0.1, 0.3 and 1 g/kg/day (0.05, 0.15, 0.5 mg tretinoin/kg/day). Possible tretinoin malformations (craniofacial abnormalities [hydrocephaly], asymmetrical thyroids, variations in ossification, and increased supernumerary ribs) were observed at maternal doses of 0.5 mg tretinoin/kg/day (approximately 2 times the MRHD based on BSA comparison and assuming 100% absorption). These findings were not observed in control animals. Other maternal and reproductive parameters in tretinoin-treated animals were not different from control. For purposes of comparison of the animal exposure to human exposure, the MRHD is defined as 4 g of ALTRENO applied daily to a 60 kg person.
Other topical tretinoin embryofetal development studies have generated equivocal results. There is evidence for malformations (shortened or kinked tail) after topical administration of tretinoin to pregnant Wistar rats during organogenesis at doses greater than 1 mg/kg/day (approximately 5 times the MRHD based on BSA comparison and assuming 100% absorption). Anomalies (humerus: short 13%, bent 6%, os parietal incompletely ossified 14%) have also been reported when 10 mg/kg/day (approximately 50 times the MRHD based on BSA comparison and assuming 100% absorption) was topically applied to pregnant rats during organogenesis. Supernumerary ribs have been a consistent finding in rat fetuses when pregnant rats were treated topically or orally with retinoids.
Oral administration of tretinoin during organogenesis has been shown to induce malformations in rats, mice, rabbits, hamsters, and nonhuman primates. Fetal malformations were observed when tretinoin was orally administered to pregnant Wistar rats during organogenesis at doses greater than 1 mg/kg/day (approximately 5 times the MRHD based on BSA comparison). In the cynomolgus monkey, fetal malformations were reported when an oral dose of 10 mg/kg/day was administered to pregnant monkeys during organogenesis (approximately 100 times the MRHD based on BSA comparison). No fetal malformations were observed at an oral dose of 5 mg/kg/day (approximately 50 times the MRHD based on BSA comparison). Increased skeletal variations were observed at all doses in this study and dose-related increases in embryo lethality and abortion were reported in this study. Similar results have also been reported in pigtail macaques.
Oral tretinoin has been shown to be fetotoxic in rats when administered at doses 10 times the MRHD based on BSA comparison. Topical tretinoin has been shown to be fetotoxic in rabbits when administered at doses 4 times the MRHD based on BSA comparison.
8.2 Lactation
Risk Summary
There are no data on the presence of tretinoin or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ALTRENO and any potential adverse effects on the breastfed child from ALTRENO.
8.4 Pediatric Use
Safety and effectiveness of ALTRENO for the topical treatment of acne vulgaris have been established in pediatric patients age 9 years to less than 17 years based on evidence from two multicenter, randomized, double-blind, parallel-group, vehicle-controlled, 12-week trials and an open-label pharmacokinetic study. A total of 318 pediatric subjects aged 9 to less than 17 years received ALTRENO in the clinical studies [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of ALTRENO in pediatric patients below the age of 9 years have not been established.
-
11 DESCRIPTION
ALTRENO (tretinoin) lotion is an opaque, pale yellow lotion containing 0.05% tretinoin by weight for topical administration.
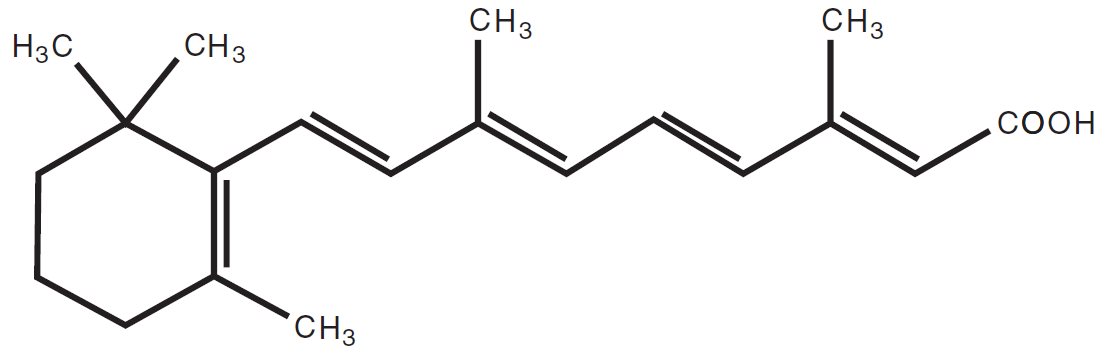
Chemically, tretinoin is all-trans-retinoic acid, also known as (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid. It is a member of the retinoid class of compounds and a metabolite of vitamin A. Tretinoin has the following chemical structure:
Molecular Formula: C20H28O2 Molecular Weight: 300.44
Each gram of ALTRENO contains 0.5 mg (0.05%) of tretinoin in an opaque, pale yellow lotion base consisting of benzyl alcohol, butylated hydroxytoluene, carbomer copolymer type B (Pemulen TR-1), carbomer homopolymer type A (Carbopol 981), glycerin, methylparaben, mineral oil, octoxynol-9, purified water, sodium hyaluronate, soluble collagen and trolamine.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tretinoin is a metabolite of vitamin A that binds with high affinity to specific retinoic acid receptors located in both the cytosol and nucleus.
Tretinoin activates three members of the retinoic acid (RAR) nuclear receptors (RARα, RARβ, and RARγ) which act to modify gene expression, subsequent protein synthesis, and epithelial cell growth and differentiation. It has not been established whether the clinical effects of tretinoin are mediated through activation of retinoic acid receptors, other mechanisms, or both.
Although the exact mode of action of tretinoin in acne treatment is unknown, current evidence suggests that topical tretinoin decreases cohesiveness of follicular epithelial cells with decreased microcomedo formation. Additionally, tretinoin stimulates mitotic activity and increased turnover of follicular epithelial cells causing extrusion of the comedones.
12.2 Pharmacodynamics
The pharmacodynamics of ALTRENO in the treatment of acne vulgaris are unknown.
12.3 Pharmacokinetics
Plasma concentrations of tretinoin and its major metabolites (isotretinoin and 4-oxo-isotretinoin) were evaluated in 20 subjects in an open-label, randomized, pharmacokinetic study. Subjects aged 10 years to less than 17 years old with acne vulgaris applied approximately 3.5 g of ALTRENO to the skin of the entire face (excluding eyes and lips), neck, upper chest, upper back and shoulders once daily for 14 days. Single-dose pharmacokinetic (PK) characteristics were determined from samples drawn on Days 1 and 2 of dosing and steady-state PK characteristics were determined from samples drawn on Days 14 and 15 under maximal use conditions. The mean baseline corrected Cmax and AUC0-t of tretinoin and its metabolites after once daily application of ALTRENO for 14 days are shown below:
Compound
Mean (±SD) Cmax
(ng/mL)Mean (±SD) AUC0-t (ng*h/mL)
Tretinoin
0.33 (0.33)
6.46 (5.15)
Isotretinoin
0.49 (0.66)
9.30 (9.95)
4-oxo-isotreinoin
0.57 (0.82)
14.51 (18.28)
The mean concentrations of tretinoin and its metabolites (isotretinoin and 4‑oxo‑isotretinoin) remain relatively stable and unchanged over the 24‑hour period after both the Day 1 dose and the Day 14 dose. Systemic concentrations of tretinoin appear to be at or near steady state by Day 14. Mean accumulation ratios of the baseline corrected AUC between Day 14 and Day 1 were 1.5, 4.5 and 7.3 for tretinoin, isotretinoin, and 4-oxo-isotretinoin, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year dermal mouse carcinogenicity study was conducted with topical administration of 0.005%, 0.025% and 0.05% of a tretinoin gel formulation. Although no drug-related tumors were observed in surviving animals, the irritating nature of the drug product precluded daily dosing, confounding data interpretation and reducing the biological significance of these results.
Studies in hairless albino mice with a different formulation suggest that concurrent exposure to tretinoin may enhance the tumorigenic potential of carcinogenic doses of UVB and UVA light from a solar simulator. This effect was confirmed in a later study in pigmented mice, and dark pigmentation did not overcome the enhancement of photocarcinogenesis by 0.05% tretinoin. Although the significance of these studies to humans is not clear, patients should minimize exposure to sunlight or artificial ultraviolet irradiation sources.
The genotoxic potential of tretinoin was evaluated in an in vitro bacterial reversion test, an in vitro chromosomal aberration assay in human lymphocytes and an in vivo rat micronucleus assay. All tests were negative.
In dermal fertility studies of another tretinoin formulation in rats, slight (not statistically significant) decreases in sperm count and motility were seen at 0.5 mg/kg/day (approximately 2 times the MRHD based on BSA comparison and assuming 100% absorption), and slight (not statistically significant) increases in the number and percent of nonviable embryos in females treated with 0.25 mg/kg/day and above (approximately the MRHD based on BSA comparison and assuming 100% absorption) were observed.
-
14 CLINICAL STUDIES
The safety and efficacy of once daily use of ALTRENO for the treatment of acne vulgaris were assessed in two multicenter, randomized, double-blind clinical trials enrolling 1640 subjects age 9 years and older with acne vulgaris. Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 non-inflammatory lesions (open and closed comedones) and two or fewer facial nodules. The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Success on the EGSS was defined as at least a 2-grade improvement from Baseline and an EGSS score of clear (0) or almost clear (1). Table 3 lists the efficacy results for trials 1 (NCT02491060) and 2 (NCT02535871).
Table 3: Efficacy Results at Week 12 Trial 1
ALTRENO
N=406Vehicle
N=414EGSS
Clear or Almost Clear and
16.5%
6.9%
2-Grade Reduction from Baseline
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
17.8
10.6
Mean Percent Reduction
47.5%
27.3%
Inflammatory Facial Lesions
Mean Absolute Reduction
13.1
10.2
Mean Percent Reduction
50.9%
40.4%
Trial 2
ALTRENO
N=413Vehicle
N=407EGSS
Clear or Almost Clear and
2-Grade Reduction from Baseline
19.8%
12.5%
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
21.9
13.9
Mean Percent Reduction
45.6%
31.9%
Inflammatory Facial Lesions
Mean Absolute Reduction
13.9
10.7
Mean Percent Reduction
53.4%
41.5%
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ALTRENO (tretinoin) lotion, 0.05% is an opaque, pale yellow topical lotion and available as:
- 45 g tube (NDC: 0187-0005-45)
- 20 g tube (NDC: 0187-0005-20)
- 50 g pump (NDC: 0187-0005-50)
Storage and Handling Conditions
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
Store pump upright.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Warn patients of the potential for skin irritation during treatment.
- Advise patients to minimize exposure to sunlight and sunlamps; recommend the use of sunscreen products and protective apparel (e.g., hat) when sun exposure cannot be avoided.
Manufactured for:
Bausch Health Americas, Inc.
Bridgewater, NJ 08807 USABy:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, CanadaU.S. Patent Number: 6,517,847
- ALTRENO is a trademark of Bausch Health Companies Inc. or its affiliates.
- © 2019 Bausch Health Companies Inc. or its affiliates
- 9650302
-
Patient Package Insert
PATIENT INFORMATION
ALTRENO™ (al-TREN-oh)
(tretinoin) lotion, 0.05%
for topical useImportant information: ALTRENO is for use on skin only. Do not use ALTRENO in your eyes, mouth, the corners of your nose, or vagina.
What is ALTRENO?
ALTRENO is a prescription medicine used on the skin (topical) to treat people with acne. Acne is a condition in which the skin has blackheads, whiteheads, and other pimples.
It is not known if ALTRENO is safe and effective in children under 9 years of age.
Before using ALTRENO, tell your healthcare provider about all your medical conditions, including if you:
- are allergic to fish. ALTRENO contains fish proteins. Tell your healthcare provider if you get hives or itching while using ALTRENO.
- have eczema or any other skin problems.
- have a sunburn.
- are pregnant or plan to become pregnant. It is not known if ALTRENO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ALTRENO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use ALTRENO?
- Use ALTRENO exactly as your healthcare provider tells you to use it.
- Apply a thin layer of ALTRENO to cover the affected areas 1 time each day.
Applying ALTRENO:
- ALTRENO comes in a tube and a pump. If you have been prescribed the:
Tube: Squeeze the lotion from the tube onto a fingertip. Apply a thin layer to cover the affected areas, as prescribed by your doctor. Spread ALTRENO evenly over the affected areas.
Pump: Fully depress the pump to dispense ALTRENO onto a fingertip. Apply a thin layer to cover the affected areas, as prescribed by your doctor. Spread ALTRENO evenly over the affected areas.
- Wash your hands after applying ALTRENO.
What should I avoid while using ALTRENO?
- You should avoid sunlamps, tanning beds, and ultraviolet light during treatment with ALTRENO.
- Minimize exposure to sunlight.
- If you have to be in the sunlight or are sensitive to sunlight, use a sunscreen with an SPF (sun protection factor) of 15 or more and wear protective clothing and a wide-brimmed hat to cover the treated areas.
What are the possible side effects of ALTRENO?
ALTRENO may cause serious side effects, including:
Skin irritation. ALTRENO may cause irritation including skin dryness, pain, redness, excessive flaking or peeling. If you develop these symptoms, your healthcare provider may tell you to use a moisturizer, decrease the number of times you apply ALTRENO, or completely stop treatment with ALTRENO. Avoid applying ALTRENO to skin that is affected by eczema or sunburned skin.
These are not all the possible side effects of ALTRENO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ALTRENO?
- Store ALTRENO at room temperature between 68° to 77°F (20° to 25°C).
- Do not freeze.
- Store ALTRENO pump upright.
Keep ALTRENO and all medicines out of the reach of children.
General information about the safe and effective use of ALTRENO
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ALTRENO for a condition for which it was not prescribed. Do not give ALTRENO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ALTRENO that is written for health professionals.
What are the ingredients in ALTRENO?
Active ingredient: tretinoin
Inactive ingredients: benzyl alcohol, butylated hydroxytoluene, carbomer copolymer type B (Pemulen TR-1), carbomer homopolymer type A (Carbopol 981), glycerin, methylparaben, mineral oil, octoxynol-9, purified water, sodium hyaluronate, soluble collagen and trolamine.
Manufactured for:
Bausch Health Americas, Inc., Bridgewater, NJ 08807 USABy:
Bausch Health Companies Inc., Laval, Quebec H7L 4A8, CanadaFor more information, call 1-800-321-4576.
U.S. Patent Number: 6,517,847
ALTRENO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2019 Bausch Health Companies Inc. or its affiliates
This Patient Information has been approved by the U.S. Food and Drug Administration.9650302
Revised: 11/2019 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Carton
-
INGREDIENTS AND APPEARANCE
ALTRENO
tretinoin lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0187-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TROLAMINE (UNII: 9O3K93S3TK) MARINE COLLAGEN, SOLUBLE (UNII: 8JC99XGU4W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-0005-45 1 in 1 CARTON 08/24/2018 1 45 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0187-0005-03 6 in 1 CARTON 08/24/2018 2 3 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 0187-0005-50 1 in 1 CARTON 04/13/2019 3 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC: 0187-0005-10 10 in 1 CARTON 05/22/2019 4 NDC: 0187-0005-04 3 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC: 0187-0005-20 1 in 1 CARTON 11/08/2019 5 20 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209353 08/24/2018 Labeler - Bausch Health US, LLC (831922488) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 245141858 MANUFACTURE(0187-0005) , PACK(0187-0005) , LABEL(0187-0005)
Trademark Results [Altreno]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALTRENO 87022985 5807207 Live/Registered |
BAUSCH HEALTH IRELAND LIMITED 2016-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.