ANTICAVITY- sodium fluoride mouthwash
Anticavity by
Drug Labeling and Warnings
Anticavity by is a Otc medication manufactured, distributed, or labeled by Publix Super Markets, Inc., Nice-Pak Products, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
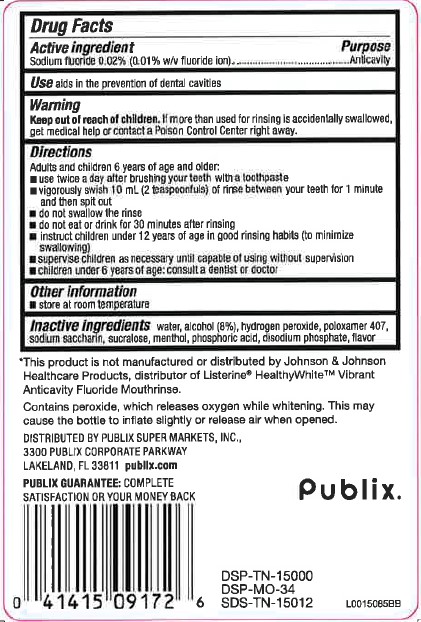
- Active Ingredient
- Use
- Warnings
- Keep out of reach of children.
-
Directions
Adults and children 6 years of age and older:
- use twice a day after brushing your teeth with a toothpaste
- vigorously swish 10 mL (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
- children under 6 years of age: consult a dentist or doctor
- Other information
- Inactive ingredients
-
Disclaimer
*This product is not manufactured or distributed by Johnson & Johnson Healthcare Products, distributor of Listerine® HealthyWhite™ Vibrant Anticavity Fluoride Mouthrinse.
Contains peroxide, which releases oxygen while whitening. This may cause the bottle to inflate slightly or release air when opened.
- ADVERSE REACTION
-
Principal Display Panel
P
Antivavity
Glowing white
Mouthrinse
- Begins working on contact
- 5 days to whiter teeth
- Helps strengthen teeth
- Kills germs that cause bad breath
*Compare to the ingredients of Listerine ®Healthywhite™ Vibrant Anticavity Fluoride Mouthrinse
Sealed with printed neckband for your protection
IMPORTANT: Read directions for proper use.
32 FL OZ (1 QT) 946 mL

-
INGREDIENTS AND APPEARANCE
ANTICAVITY
sodium fluoride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 56062-435 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) HYDROGEN PEROXIDE (UNII: BBX060AN9V) POLOXAMER 407 (UNII: TUF2IVW3M2) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SUCRALOSE (UNII: 96K6UQ3ZD4) MENTHOL (UNII: L7T10EIP3A) PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 56062-435-45 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/15/2015 2 NDC: 56062-435-43 474 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/15/2015 10/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 09/15/2015 Labeler - Publix Super Markets, Inc. (006922009) Registrant - Nice-Pak Products, LLC (119091520) Establishment Name Address ID/FEI Business Operations Nice-Pak Products, LLC 119091514 manufacture(56062-435)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.