Sepia by Energique, Inc. / Apotheca Company DRUG FACTS:
Sepia by
Drug Labeling and Warnings
Sepia by is a Homeopathic medication manufactured, distributed, or labeled by Energique, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SEPIA 30C- sepia liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
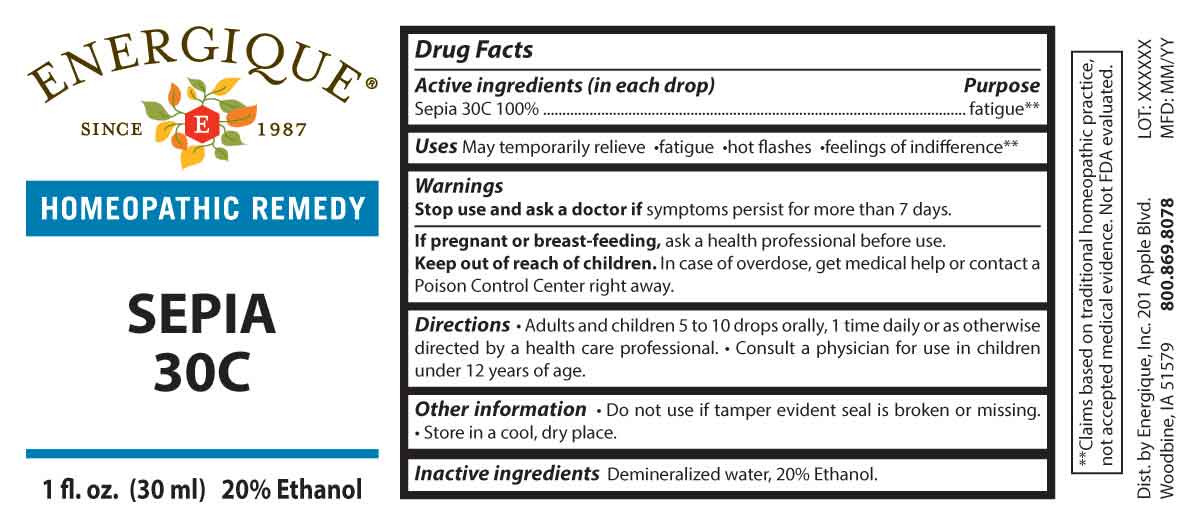
DRUG FACTS:
PURPOSE:
Sepia - fatigue**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
USES:
May temporarily relieve fatigue hot flashes feeling of indifference**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
Stop use and ask a doctor if symptoms persist for more than 7 days. If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
| SEPIA
30C
sepia liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0698) , api manufacture(44911-0698) , label(44911-0698) , pack(44911-0698) | |
Trademark Results [Sepia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SEPIA 98718916 not registered Live/Pending |
Karno Sound Limited 2024-08-27 |
 SEPIA 98002469 not registered Live/Pending |
CIRCA SCIENTIFIC, INC. 2023-05-18 |
 SEPIA 97878943 not registered Live/Pending |
Changsha Sibiya Technology Co., Ltd. 2023-04-07 |
 SEPIA 97480605 not registered Live/Pending |
Changsha Sibiya Technology Co., Ltd. 2022-06-29 |
 SEPIA 97251720 not registered Live/Pending |
Lesaffre et Compagnie 2022-02-03 |
 SEPIA 97178048 not registered Live/Pending |
Ambeauty 2021-12-17 |
 SEPIA 90832350 not registered Live/Pending |
Sepia Painting and Finishing Inc. 2021-07-16 |
 SEPIA 88690634 not registered Live/Pending |
Nido Surgical, Inc. 2019-11-13 |
 SEPIA 87926064 5741927 Live/Registered |
HLC Holding, Inc. 2018-05-17 |
 SEPIA 86091571 4537392 Live/Registered |
Sepia Ink Block LLC 2013-10-15 |
 SEPIA 79297591 not registered Live/Pending |
LESAFFRE ET COMPAGNIE 2020-09-29 |
 SEPIA 78572881 3057739 Dead/Cancelled |
Black Entertainment Television, Inc. 2005-02-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.