EMERPHED -PFS- ephedrine sulfate injection
Emerphed by
Drug Labeling and Warnings
Emerphed by is a Prescription medication manufactured, distributed, or labeled by Nexus Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EMERPHED and EMERPHED-PFS safely and effectively. See full prescribing information for EMERPHED and EMERPHED-PFS.
EMERPHED® (ephedrine sulfate) injection, for intravenous use.
EMERPHED®-PFS (ephedrine sulfate injection), for intravenous use.
Initial U.S. Approval: 2016INDICATIONS AND USAGE
EMERPHED and EMERPHED-PFS is an alpha- and beta- adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia. (1)
DOSAGE AND ADMINISTRATION
- This is a premixed formulation. Do not dilute prior to use.
- Discard any unused portion of EMERPHED or EMERPHED-PFS.
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- EMERPHED and EMERPHED-PFS is a clear, colorless solution. Do not use if the solution is not clear or if particulate matter is present.
- The single-dose prefilled syringe is intended for use in one patient during one surgical procedure.
DOSAGE FORMS AND STRENGTHS
Injection: 50 mg/10 mL ephedrine sulfate, equivalent to 38 mg/10 mL ephedrine base in a single-dose vial (5 mg/ mL ephedrine sulfate, equivalent to 3.8 mg/mL ephedrine base); a 25 mg/5 mL (5 mg/mL) ephedrine sulfate in a 5 mL single-dose prefilled syringe; and a 50 mg/10 mL (5 mg/mL) ephedrine sulfate in a 10 mL single-dose prefilled syringe. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions during treatment : nausea, vomiting, and tachycardia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Nexus Pharmaceuticals at (855) 642-2594 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Interactions that Augment Pressor Effect: clonidine, oxytocin and oxytocic drugs, propofol, monoamine oxidase inhibitors (MAOIs), and atropine. Monitor blood pressure. (7)
- Interactions that Antagonize the Pressor Effect: Antagonistic effects with α-adrenergic antagonists, β-adrenergic antagonists, reserpine, quinidine, mephentermine. Monitor blood pressure. (7)
- Guanethidine: EMERPHED and EMERPHED-PFS may inhibit the neuron blockage produced by guanethidine, resulting in loss of antihypertensive effectiveness. Monitor blood pressure and adjust the dosage of pressor accordingly.
- Rocuronium: EMERPHED and EMERPHED-PFS may reduce the onset time of neuromuscular blockade when used for intubation with rocuronium if administered simultaneously with anesthetic induction. Be aware of this potential interaction. No treatment or other interventions are needed.
- Epidural anesthesia: EMERPHED and EMERPHED-PFS may decrease the efficacy of epidural blockade by hastening the regression of sensory analgesia. Monitor and treat the patient according to clinical practice.
- Theophylline: Concomitant use of EMERPHED and EMERPHED-PFS may increase the frequency of nausea, nervousness, and insomnia. Monitor patient for worsening symptoms and manage symptoms according to clinical practice.
- Cardiac glycosides: Giving EMERPHED and EMERPHED-PFS with a cardiac glycoside, such as digitalis, may increase the possibility of arrhythmias. Carefully monitor patients on cardiac glycosides who are also administered EMERPHED and EMERPHED-PFS.
Revised: 9/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
2.2 Dosing for the Treatment of Clinically Important Hypotension in the Setting of Anesthesia
2.3 Instructions for Use of Prefilled Syringe
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Pressor Effect with Concomitant Oxytocic Drugs
5.2 Tolerance and Tachyphylaxis
5.3 Risk of Hypertension When Used Prophylactically
6. ADVERSE REACTIONS
7. DRUG INTERACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
- This is a premixed formulation. Do not dilute prior to use.
- Discard any unused portion of EMERPHED.
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- The single-dose prefilled syringe is intended for use in one patient during one surgical procedure.
EMERPHED is a clear, colorless solution. Do not use if the solution is not clear or if particulate matter is present.
2.2 Dosing for the Treatment of Clinically Important Hypotension in the Setting of Anesthesia
The recommended dosages for the treatment of clinically important hypotension in the setting of anesthesia is an initial dose of 5 mg to 10 mg administered by intravenous bolus. Administer additional boluses as needed, not to exceed a total dosage of 50 mg.
- Adjust dosage according to the blood pressure goal (i.e., titrate to effect).
2.3 Instructions for Use of Prefilled Syringe
The proper method of administration of EMERPHED-PFS injection is described in the following instructions.
For each single-dose prefilled syringe:
- Remove syringe from tray and check that it is not damaged or leaking.
- Inspect drug product in glass syringe for any visible particulate matter or discoloration prior to use. Discard if particulate matter or discoloration is present.
- Do not remove the tamper evident seal. Push plunger rod slightly in to break the stopper loose while tip cap is still on.
- Hold the syringe upright on the syringe barrel (C). With the other hand, take hold of the cap (A) and twist off cap counterclockwise from syringe tip (see Figure 1).
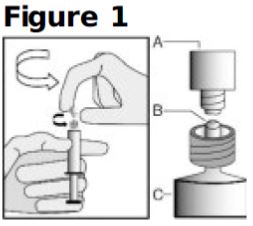
- Once cap (A) is off, DO NOT TOUCH THE STERILE SYRINGE TIP (Luer-Lok) (B) (see Figure 2).
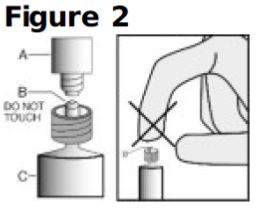
- Discard the tip cap.
- Expel air bubble.
- Adjust dose into sterile material (if applicable).
- Connect the syringe to an appropriate intravenous connection.
- Before injection, ensure that the syringe is securely attached to the needle or needleless luer access device (NLAD).
- Depress plunger rod to deliver medication. Ensure that pressure is maintained on the plunger rod during the entire administration.
- Remove syringe from NLAD (if applicable) and discard into appropriate receptacle.
- To prevent needle stick injuries, do not recap needle when needle is connected to syringe.
NOTE: All steps must be done sequentially
- Do not re-sterilize syringe
- Do not use this product on a sterile field
- Do not introduce any other fluid into the syringe at any time
- This product is for single dose only
-
3. DOSAGE FORMS AND STRENGTHS
EMERPHED (ephedrine sulfate injection) is a clear, colorless solution available as:
- A single-dose vial that contains 50 mg/10 mL (5 mg/mL) ephedrine sulfate, equivalent to 38 mg/10 mL (3.8 mg/mL) ephedrine base.
EMERPHED-PFS (ephedrine sulfate injection) is a clear, colorless solution available as:
- A single-dose 5 mL prefilled syringe that contains 25 mg/5 mL (5 mg/mL) ephedrine sulfate, equivalent to 19 mg/5 mL (3.8 mg/mL) ephedrine base.
- A single-dose pre-filled syringe that contains 50 mg/10 mL (5 mg/mL) ephedrine sulfate, equivalent to 38 mg/10 mL (3.8 mg/mL) ephedrine base.
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Pressor Effect with Concomitant Oxytocic Drugs
Serious postpartum hypertension has been described in patients who received both a vasopressor (i.e., methoxamine, phenylephrine, ephedrine) and an oxytocic (i.e., methylergonovine, ergonovine) [see Drug Interactions (7)]. Some of these patients experienced a stroke. Carefully monitor the blood pressure of individuals who have received both EMERPHED and EMERPHED-PFS and an oxytocic.
5.2 Tolerance and Tachyphylaxis
Data indicate that repeated administration of ephedrine can result in tachyphylaxis. Be aware of this possibility when treating anesthesia-induced hypotension with EMERPHED and EMERPHED-PFS and be prepared with an alternative pressor to mitigate unacceptable responsiveness.
-
6. ADVERSE REACTIONS
The following adverse reactions associated with the use of ephedrine sulfate were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Gastrointestinal disorders: Nausea, vomiting
Cardiac disorders: Tachycardia, palpitations (thumping heart), reactive hypertension, bradycardia, ventricular ectopics, R-R variability
Nervous system disorders: Dizziness
Psychiatric disorders: Restlessness
-
7. DRUG INTERACTIONS
Interactions that Augment the Pressor Effect Oxytocin and oxytocic drugs Clinical Impact: Serious postpartum hypertension has been described in patients who received both a vasopressor (i.e., methoxamine, phenylephrine, ephedrine) and an oxytocic (i.e., methylergonovine, ergonovine). Some of these patients experienced a stroke. Intervention: Carefully monitor the blood pressure of individuals who have received both EMERPHED and EMERPHED-PFS and an oxytocic. Clonidine, propofol, monoamine oxidase inhibitors (MAOIs ), atropine Clinical Impact: These drugs augment the pressor effect of ephedrine. Intervention: Carefully monitor the blood pressure of individuals who have received both EMERPHED and EMERPHED-PFS and any of these drugs. Drugs that Antagonize the Pressor Effect α-adrenergic antagonists, β-adrenergic receptor antagonists, reserpine, quinidine, mephentermine Clinical Impact: These drugs antagonize the pressor effect of ephedrine. Intervention: Carefully monitor the blood pressure of individuals who have received both EMERPHED and EMERPHED-PFS and any of these drugs. Other Drug Interactions Guanethidine Clinical Impact: EMERPHED and EMERPHED-PFS may inhibit the neuron blockage produced by guanethidine, resulting in loss of antihypertensive effectiveness. Intervention: Clinician should monitor patient for blood pressor response and adjust the dosage or choice of pressor accordingly. Rocuronium Clinical Impact: EMERPHED and EMERPHED-PFS may reduce the onset time of neuromuscular blockade when used for intubation with rocuronium if administered simultaneously with anesthetic induction. Intervention: Be aware of this potential interaction. No treatment or other interventions are needed. Epidural anesthesia Clinical Impact: EMERPHED and EMERPHED-PFS may decrease the efficacy of epidural blockade by hastening the regression of sensory analgesia. Intervention: Monitor and treat the patient according to clinical practice. Theophylline Clinical Impact: Concomitant use of EMERPHED and EMERPHED-PFS may increase the frequency of nausea, nervousness, and insomnia. Intervention: Monitor patient for worsening symptoms and manage symptoms according to clinical practice. Cardiac glycosides Clinical Impact: Giving EMERPHED and EMERPHED-PFS with a cardiac glycoside, such as digitalis, may increase the possibility of arrhythmias. Intervention: Carefully monitor patients on cardiac glycosides who are also administered ephedrine. -
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from randomized studies, case series, and reports of ephedrine sulfate use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, there are clinical considerations due to underlying conditions [see Clinical Considerations]. In animal reproduction studies, decreased fetal survival and fetal body weights were observed in the presence of maternal toxicity after normotensive pregnant rats were administered 60 mg/kg intravenous ephedrine sulfate (12 times the maximum recommended human dose (MRHD) of 50 mg/day). No malformations or embryofetal adverse effects were observed when pregnant rats or rabbits were treated with intravenous bolus doses of ephedrine sulfate during organogenesis at doses 1.9 and 7.7 times the MRHD, respectively [See data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Untreated hypotension associated with spinal anesthesia for cesarean section is associated with an increase in maternal nausea and vomiting. A decrease in uterine blood flow due to maternal hypotension may result in fetal bradycardia and acidosis.
Fetal/Neonatal Adverse Reactions
Cases of potential metabolic acidosis in newborns at delivery with maternal ephedrine exposure have been reported in the literature. These reports describe umbilical artery pH of ≤7.2 at the time of delivery [see Clinical Pharmacology 12.3]. Monitoring of the newborn for signs and symptoms of metabolic acidosis may be required. Monitoring of infant's acid-base status is warranted to ensure that an episode of acidosis is acute and reversible.
Data
Animal Data
Decreased fetal body weights were observed when pregnant rats were administered intravenous bolus doses of 60 mg/kg ephedrine sulfate (12 times the maximum recommended human dose (MRHD) of 50 mg based on body surface area) from Gestation Day 6-17. This dose was associated with evidence of maternal toxicity (decreased body weight of dams and abnormal head movements). No malformations or fetal deaths were noted at this dose. No effects on fetal body weight were noted at 10 mg/kg (1.9 times the MRHD of 50 mg).
No evidence of malformations or embryo-fetal toxicity were noted in pregnant rabbits administered intravenous bolus doses up to 20 mg/kg ephedrine sulfate (7.7 times the maximum recommended human dose (MRHD) of 50 mg based on body surface area) from Gestation Day 6-20. This dose was associated with expected pharmacological maternal effects (increased respiration rate, dilated pupils, piloerection).
Decreased fetal survival and body weights in the presence of maternal toxicity (increased mortality) were noted when pregnant dams were administered intravenous bolus doses of 60 mg/kg epinephrine sulfate (approximately 12 times the MRHD based on body surface area) from GD 6 through Lactation Day 20. No adverse effects were noted at 10 mg/kg (1.9 times the MRHD).
8.2 Lactation
Risk Summary
A single published case report indicates that ephedrine is present in human milk. However, no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EMERPHED or EMERPHED-PFS and any potential adverse effects on the breastfed child from EMERPHED and EMERPHED-PFS or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of EMERPHED and EMERPHED-PFS in pediatric patients have not been established.
Animal Toxicity Data
In a study in which juvenile rats were administered intravenous bolus doses of 2, 10, or 60 mg/kg ephedrine sulfate daily from Postnatal Day 35 to 56, an increased incidence of mortality was noted at the high dose of 60 mg/kg. The no-adverse-effect level was 10 mg/kg (approximately 1.9 times a maximum daily dose of 50 mg in a 60 kg person based on body surface area)
8.5 Geriatric Use
Clinical studies of ephedrine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
Ephedrine and its metabolite are excreted in urine. In patients with renal impairment, excretion of ephedrine is likely to be affected with a corresponding increase in elimination half-life, which will lead to slow elimination of ephedrine and consequently prolonged pharmacological effect and potentially adverse reactions. Monitor patients with renal impairment carefully after the initial bolus dose for adverse events.
-
10. OVERDOSAGE
Overdose of EMERPHED and EMERPHED-PFS can cause a rapid rise in blood pressure. In the case of an overdose, careful monitoring of blood pressure is recommended. If blood pressure continues to rise to an unacceptable level, parenteral antihypertensive agents can be administered at the discretion of the clinician.
-
11. DESCRIPTION
EMERPHED / EMERPHED-PFS (ephedrine sulfate injection) is a clear, colorless, sterile solution for intravenous injection. The chemical name of ephedrine sulfate is benzenemethanol, α-[1-(methylamino)ethyl]-, [R-(R*,S*)]-, sulfate (2:1) (salt), and the molecular weight is 428.5 g/mol. Its structural formula is depicted below:

Ephedrine sulfate is freely soluble in water and ethanol, very slightly soluble in chloroform, and practically insoluble in ether. Each mL contains ephedrine sulfate, USP 5 mg (equivalent to 3.8 mg ephedrine base), 0.9% sodium chloride, USP in water for injection. The pH range is 4.5 to 7.0.
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ephedrine sulfate is a sympathomimetic amine that directly acts as an agonist at α- and β-adrenergic receptors and indirectly causes the release of norepinephrine from sympathetic neurons. Pressor effects by direct alpha- and beta-adrenergic receptor activation are mediated by increases in arterial pressures, cardiac output, and peripheral resistance. Indirect adrenergic stimulation is caused by norepinephrine release from sympathetic nerves.
12.2 Pharmacodynamics
Ephedrine stimulates heart rate and cardiac output and variably increases peripheral resistance; as a result, ephedrine usually increases blood pressure. Stimulation of the α-adrenergic receptors of smooth muscle cells in the bladder base may increase the resistance to the outflow of urine. Activation of β- adrenergic receptors in the lungs promotes bronchodilation.
The overall cardiovascular effect from ephedrine is the result of a balance among α-1 adrenoceptor- mediated vasoconstriction, β-2 adrenoceptor- mediated vasoconstriction, and β-2 adrenoceptor-mediated vasodilatation. Stimulation of the β-1 adrenoceptors results in positive inotrope and chronotrope action.
Tachyphylaxis to the pressor effects of ephedrine may occur with repeated administration [see Warnings and Precautions 5.2].
12.3 Pharmacokinetics
Publications studying pharmacokinetics of oral administration of (-)-ephedrine support that (-)-ephedrine is metabolized into norephedrine. However, the metabolism pathway is unknown. Both the parent drug and the metabolite are excreted in urine. Limited data after IV administration of ephedrine support similar observations of urinary excretion of drug and metabolite. The plasma elimination half-life of ephedrine following oral administration was about 6 hours.
Ephedrine crosses the placental barrier [see Use in Specific Populations 8.1].
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Two-year feeding studies in rats and mice conducted under the National Toxicology Program (NTP) demonstrated no evidence of carcinogenic potential with ephedrine sulfate at doses up to 10 mg/kg/day and 27 mg/kg/day (approximately 2 times and 3 times the maximum human recommended dose on a mg/m2 basis, respectively).
Mutagenesis: Ephedrine sulfate tested negative in the in vitro bacterial reverse mutation assay, the in vitro mouse lymphoma assay, the in vitro sister chromatid exchange, the in vitro chromosomal aberration assay, and the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility: There was no impact on fertility or early embryonic development in a study in which male rats were administered intravenous bolus doses of 0, 2, 10, or 60 mg/kg ephedrine sulfate (up to 12 times the maximum recommended human dose of 50 mg based on body surface area) for 28 days prior to mating and through gestation and females were treated for 14 days prior to mating through Gestation Day 7.
-
14. CLINICAL STUDIES
The evidence for the efficacy of EMERPHED / EMERPHED-PFS (ephedrine sulfate injection) is derived from the published literature. Increases in blood pressure following administration of ephedrine were observed in 14 studies, including 9 where ephedrine was used in pregnant women undergoing neuraxial anesthesia during Cesarean delivery, 1 study in non-obstetric surgery under neuraxial anesthesia, and 4 studies in patients undergoing surgery under general anesthesia. Ephedrine has been shown to raise systolic and mean blood pressure when administered as a bolus dose following the development of hypotension during anesthesia.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
EMERPHED (ephedrine sulfate injection) is a clear, colorless solution available as single-dose vials containing 5 mg/mL ephedrine sulfate, equivalent to 3.8 mg/mL ephedrine base and is supplied as follows:
NDC Presentation 14789-250-07 10 mL clear glass, single-dose vial; strength 50 mg/10 mL (5 mg/mL) 14789-250-10 10 mL vials packaged in a carton of 10 EMERPHED-PFS (ephedrine sulfate injection) is a clear, colorless solution available as single-dose pre-filled syringes containing 5 mg/mL ephedrine sulfate, equivalent to 3.8 mg/mL ephedrine base and is supplied as follows:
NDC Presentation 14789-251-09 5 mL clear glass, single-dose pre-filled syringe; strength 25 mg/5 mL (5 mg/mL) 14789-251-10 5 mL pre-filled syringes packaged in a carton of 10 14789-252-09 10 mL clear glass, single-dose pre-filled syringe; strength 50 mg/10 mL (5 mg/mL) 14789-252-10 10 mL pre-filled syringes packaged in a carton of 10 The single-dose prefilled syringes, each fitted with a removable tip cap and plunger rods, are presented in a carton.
EMERPHED (ephedrine sulfate injection) 50 mg/10 mL (5 mg/mL) and EMERPHED-PFS (ephedrine sulfate injection) 50 mg/10 mL (5 mg/mL) and 25 mg/5 mL (5 mg/mL) are not made with natural rubber latex.
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store in carton until time of use. For single dose only. Discard unused portion.
The single-dose prefilled syringe is intended for use in one patient during one surgical procedure.
Patent, www.emerphed.com/patent
Manufactured in Italy for:

Nexus Pharmaceuticals, LLC
400 Knightsbridge Parkway
Lincolnshire, IL 60069
USA
EPFPI01ITR06
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 5 mg/mL Carton Label
NDC 14789-252-10
Rx Only
EMERPHED®-PFS
(ephedrine sulfate injection)
50 mg/10 mL (5 mg/mL)
(equivalent to 38 mg/10 mL ephedrine base)Premixed formulation -Do not dilute
For Intravenous Use Only
10 x 10 mL Single-dose Prefilled Syringes
NEXUS
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 5 mg/mL Vial Label
NDC: 14789-252-09
Rx Only
EMERPHED®-EPF
(ephedrine sulfate injection)
50 mg/10 mL (5 mg/mL)
(equivalent to 38 mg/5 mL ephedrine base)Premixed formulation –Do not dilute
For Intravenous Use Only
10 mL Single-dose Prefilled Syringe

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 25 mg/5 mL Carton Label
NDC 14789-251-10
Rx Only
EMERPHED®-EPF
(ephedrine sulfate injection)
25 mg/5 mL (5 mg/mL)
(equivalent to 19 mg/5 mL ephedrine base)Premixed formulation -Do not dilute
For Intravenous Use Only
10 x 5 mL Single-dose Prefilled Syringes
NEXUS
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 25 mg/5 mL Vial Label
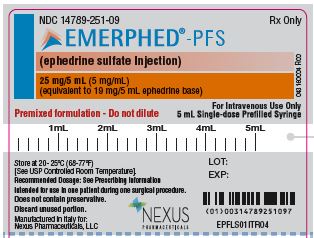
NDC: 14789-251-09
Rx Only
EMERPHED®-EPF
(ephedrine sulfate injection)
25 mg/5 mL (5 mg/mL)
(equivalent to 19 mg/5 mL ephedrine base)Premixed formulation –Do not dilute
For Intravenous Use Only
5 mL Single-dose Prefilled Syringe
-
INGREDIENTS AND APPEARANCE
EMERPHED -PFS
ephedrine sulfate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 14789-252 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ephedrine Sulfate (UNII: U6X61U5ZEG) (EPHEDRINE - UNII:GN83C131XS) Ephedrine Sulfate 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14789-252-10 10 in 1 CARTON 03/01/2023 1 NDC: 14789-252-09 10 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213407 03/01/2023 EMERPHED -PFS
ephedrine sulfate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 14789-251 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ephedrine Sulfate (UNII: U6X61U5ZEG) (EPHEDRINE - UNII:GN83C131XS) Ephedrine Sulfate 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14789-251-10 10 in 1 CARTON 03/01/2023 1 NDC: 14789-251-09 5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213407 03/01/2023 Labeler - Nexus Pharmaceuticals, LLC (620714787) Establishment Name Address ID/FEI Business Operations Nexus Pharmaceuticals, LLC 620714787 ANALYSIS(14789-252, 14789-251)
Trademark Results [Emerphed]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EMERPHED 88880799 not registered Live/Pending |
Nexus Pharmaceuticals, Inc. 2020-04-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.