Acetaminophen 325 mg TabletsRegular Strength
ACETAMINOPHEN by
Drug Labeling and Warnings
ACETAMINOPHEN by is a Otc medication manufactured, distributed, or labeled by Akron Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
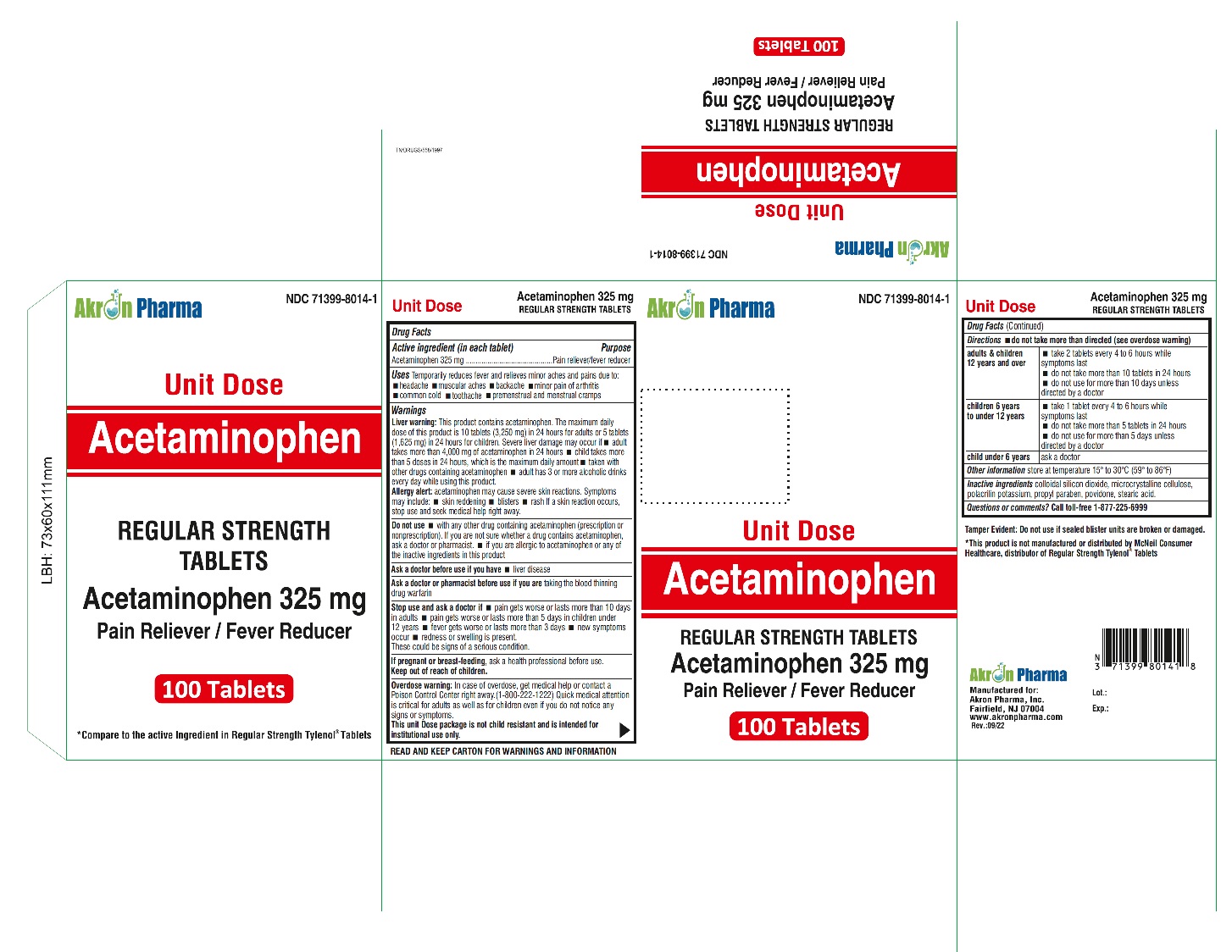
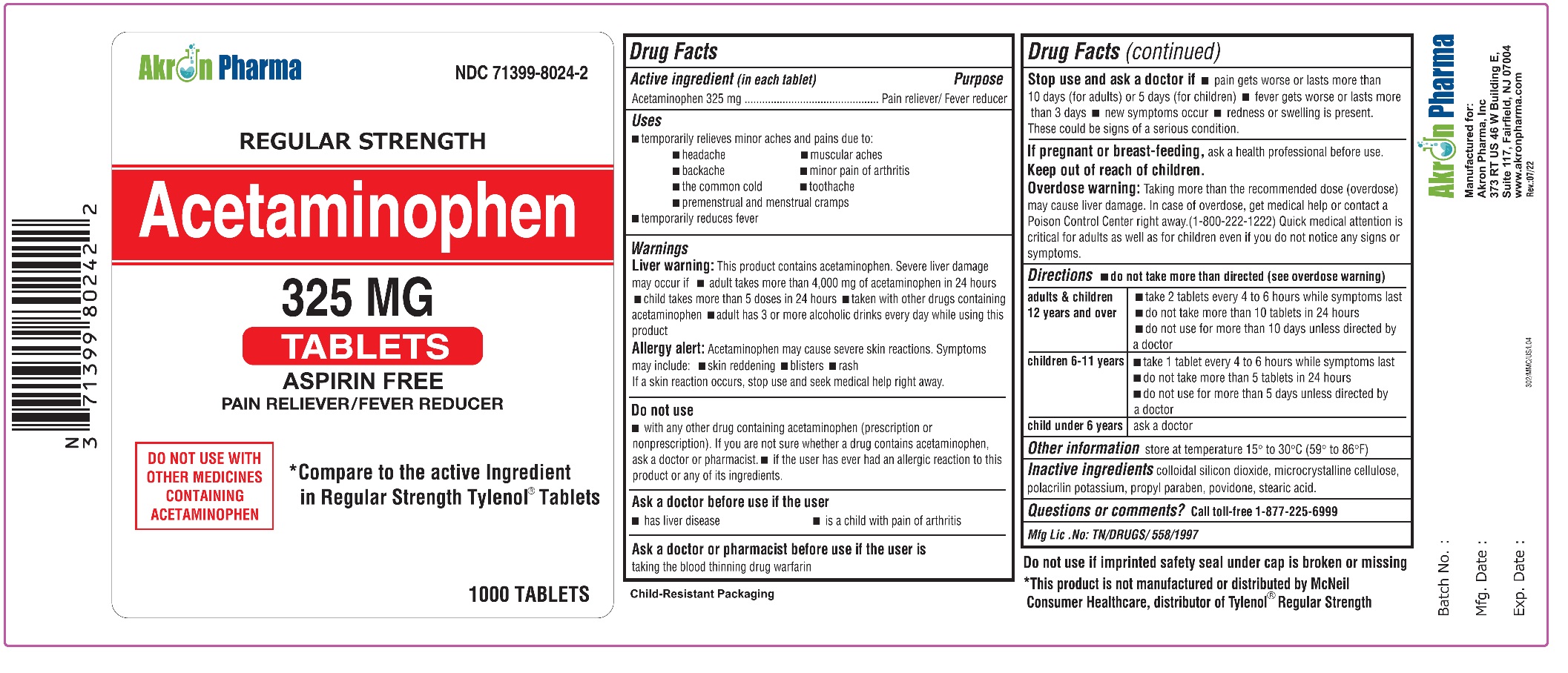
ACETAMINOPHEN- acetaminophen tablet
Akron Pharma Inc.
----------
Acetaminophen 325 mg Tablets
Regular Strength
Uses
To reduce fever and for the temporary relief of minor aches and pains due to:
headache
muscular aches
backache
minor pain of arthritis
the common cold
toothache
premenstrual and menstrual cramps.
Temporarily reduces fever.
Warnings
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if the user has ever had an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if:
- pain gets worse or lasts more than 10 days (for adults) or 5 days (for children)
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present.
These could be signs of a serious condition.
Keep out of reach of children.
Overdose Warning:
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away.(1-800-222-1222)
Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions - do not take more than directed (see overdose warning)
| adults & children 12 years and over |
|
| children 6-11 years |
|
| child under 6 years | ask a doctor |
Inactive Ingredients:
colloidal silicon dioxide, microcrystalline cellulose, polacrilin potassium, propylparaben, povidone, stearic acid.
Questions or Comments?
Call toll-free 1-877-225-6999
Manufactured for
Akron Pharma, Inc.,
373 RT US46 W Building E,
Suite 117, Fairfeld, NJ - 07004
Mfg. Lic. No: TN/DRUGS/558/1997
* This product is not manufactured or distributed by Johnson and Johnson, consumer inc., distributor of regular Tylenol Tablets.
| ACETAMINOPHEN
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ACETAMINOPHEN
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akron Pharma Inc. (067878881) |
Trademark Results [ACETAMINOPHEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.