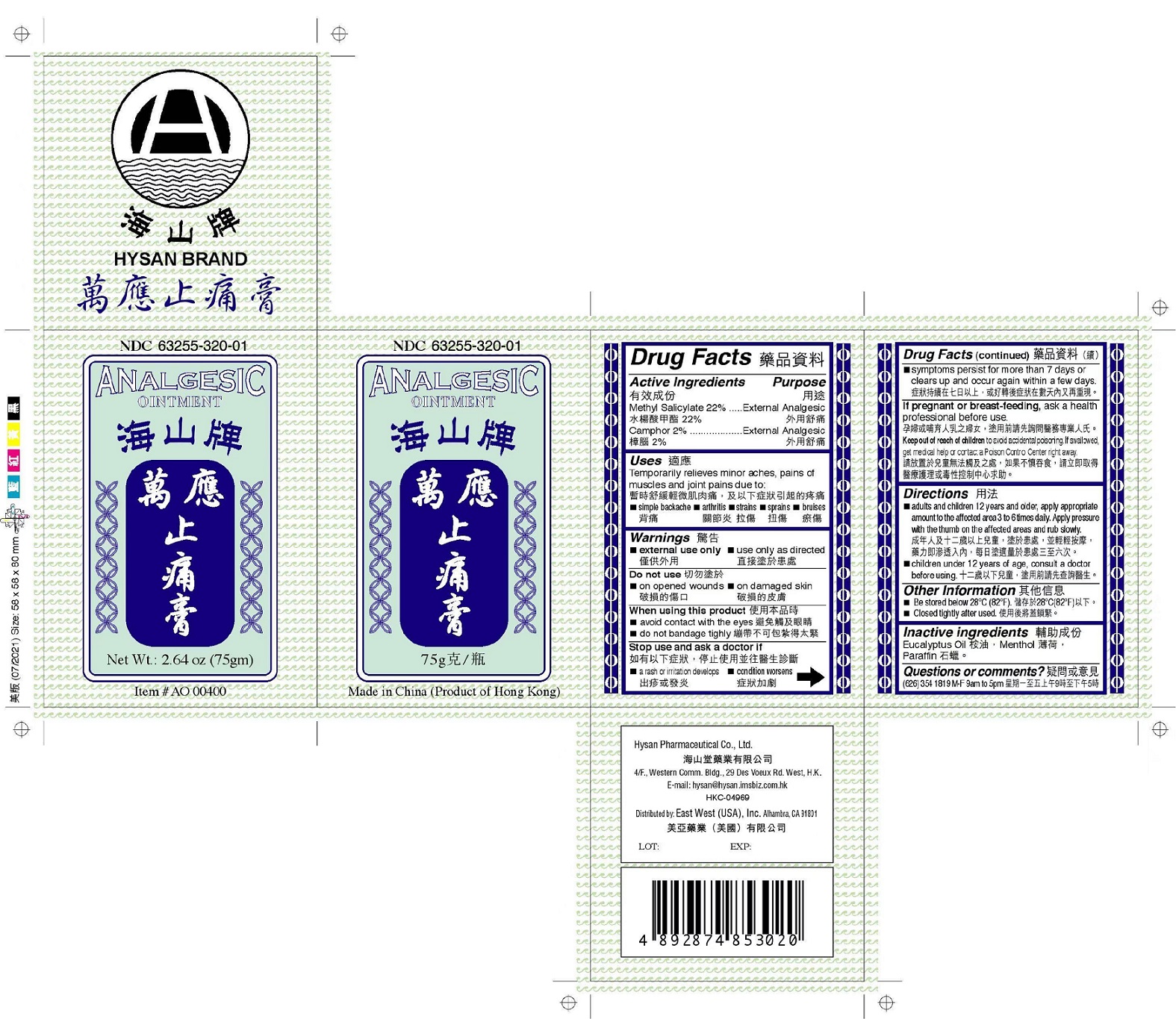
HYSAN ANALGESIC by HYSAN PHARMACEUTICAL CO LTD
HYSAN ANALGESIC by
Drug Labeling and Warnings
HYSAN ANALGESIC by is a Otc medication manufactured, distributed, or labeled by HYSAN PHARMACEUTICAL CO LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYSAN ANALGESIC- methyl salicylate, camphor (synthetic) ointment
HYSAN PHARMACEUTICAL CO LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Temporarily relieves minor aches, pains of muscles and joint pains due to:
- simple backache
- arthritis
- strains
- sprains
- bruises
Stop use and ask a doctor if
- a rash or irritation develops
- condition worsens
- symptoms persist for more than 7 days or clears up and occur again within a few days
Keep out of reach of children to avoid accidental poisoning. If swallowed, get medical help or contact a Poison Control Center right away
| HYSAN ANALGESIC
methyl salicylate, camphor (synthetic) ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - HYSAN PHARMACEUTICAL CO LTD (666427133) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HYSAN PHARMACEUTICAL CO LTD | 666427133 | manufacture(63255-320) | |
Revised: 7/2021
Document Id: a62982b1-c3e7-4e92-8a38-a9c3c58d764e
Set id: 1486357e-c17f-45e3-a5ad-900ded791dfe
Version: 2
Effective Time: 20210728