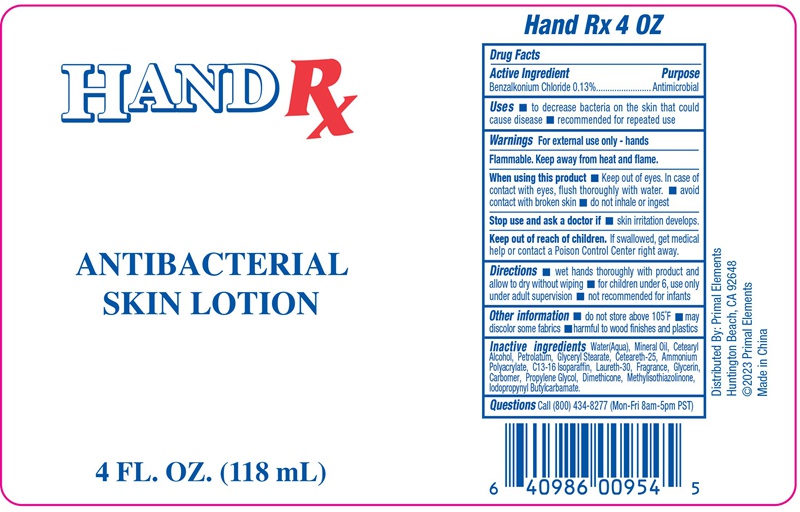
ANTIBACTERIAL SKIN by NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD.
ANTIBACTERIAL SKIN by
Drug Labeling and Warnings
ANTIBACTERIAL SKIN by is a Otc medication manufactured, distributed, or labeled by NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL SKIN- benzalkonium chloride lotion
NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD.
----------
When using this product
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- avoid contact with broken skin
- do not inhale or ingest
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wet hands thoroughly with product and allow to dry without wiping
- for children under 6, use only under adult supervision
- not recommended for infants
Other information
- do not store above 105℉
- may discolor some fabrics
- harmful to wood finishes and plastics
| ANTIBACTERIAL SKIN
benzalkonium chloride lotion |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD. (530766098) |
Revised: 7/2024
Document Id: 31361f23-43f9-36f7-e063-6294a90a1baa
Set id: 14889d9c-b201-4fc1-e063-6394a90a7e8e
Version: 2
Effective Time: 20240701
NINGBO
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.