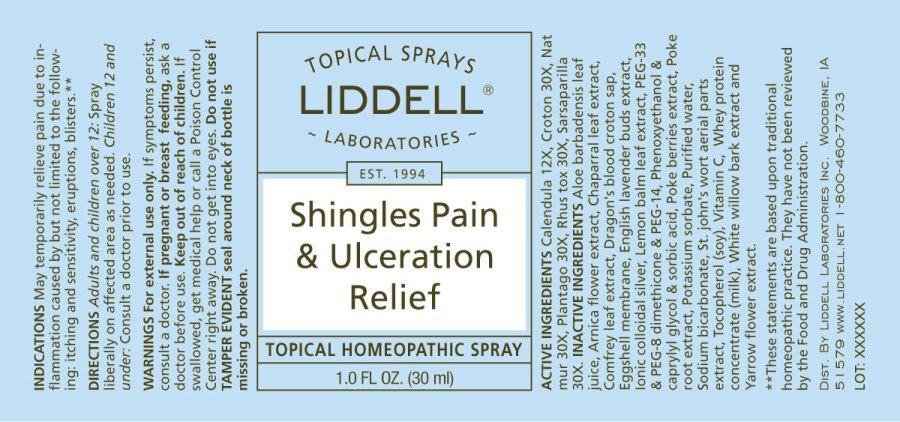
SHINGLES PAIN ULCERATION RELIEF by Liddell Laboratories, Inc. / Apotheca Company DRUG FACTS:
SHINGLES PAIN ULCERATION RELIEF by
Drug Labeling and Warnings
SHINGLES PAIN ULCERATION RELIEF by is a Homeopathic medication manufactured, distributed, or labeled by Liddell Laboratories, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SHINGLES PAIN ULCERATION RELIEF- calendula officinalis, croton tiglium, natrum muriaticum, plantago major, rhus tox, sarsaparilla (smilax regelii) spray
Liddell Laboratories, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DRUG FACTS:
ACTIVE INGREDIENTS:
CALENDULA OFFICINALIS 12X, CROTON TIGLIUM 30X, NATRUM MURIATICUM 30X, PLANTAGO MAJOR 30X, RHUS TOX 30X, SARSAPRILLA (SMILAX REGELII) 30X
INDICATIONS:
May temporarily relieve pain due to inflammation caused by but not limited to the following: itching and sensitivity, eruptions, blisters.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
For external use only.
If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Do not get into eyes.
Do not use if TAMPER EVIDENT seal is broken or missing.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children over 12: Spray liberally on affected area as needed. Children 12 and under: Consult a doctor prior to use.
INDICATIONS:
May temporarily relieve pain due to inflammation caused by but not limited to the following: itching and sensitivity, eruptions, blisters.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
INACTIVE INGREDIENTS:
ALOE BARBADENSIS LEAF JUICE (ALOE VERA 10X CONCENTRATE), ARNICA MONTANA (FLOWER), LARREA TRIDENTATA (LEAF), SYMPHYTUM OFFICINALE (LEAF), CROTON LECHLERI (SAP), INSTANTIZED SOLUBLE EGGSHELL MEMBRANE + (BIOVADERM BRAND WITH 1% SUNFLOWER LECITHIN), LAVANDULA ANGUSTIFOLIA (BUDS), IONIC COLLOIDAL SILVER, MELISSA OFFICINALIS (LEAF), [PEG-33, PEG-8 DIMETHICONE, PEG-14 (SILSENSE COPOLYOL-1 SILICONE)], [PHENOXYETHANOL, CAPRYLYL GLYCOL, SORBIC ACID (OPTIPHEN-PLUS)], PHYTOLACCA AMERICANA (BERRIES), PHYTOLACCA AMERICANA (ROOT), POTASSIUM SORBATE, PURIFIED WATER, SODIUM BICARBONATE, HYPERICUM PERFORATUM (AERIAL PARTS), TOCOPHEROL [VITAMIN E OIL](DERIVED FROM SOY BEAN), VITAMIN C POWDER (ASCORBIC ACID), WHEY PROTEIN CONCENTRATE, SALIX ALBA (BARK), ACHILLEA MILLEFOLIUM (FLOWER)
| SHINGLES PAIN ULCERATION RELIEF
calendula officinalis, croton tiglium, natrum muriaticum, plantago major, rhus tox, sarsaparilla (smilax regelii) spray |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Liddell Laboratories, Inc. (832264241) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(50845-0215) , api manufacture(50845-0215) , label(50845-0215) , pack(50845-0215) | |