KETOTIFEN FUMARATE solution/ drops
Ketotifen Fumarate by
Drug Labeling and Warnings
Ketotifen Fumarate by is a Otc medication manufactured, distributed, or labeled by KAISER FOUNDATION HOSPITALS, Akorn AG, Akorn, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
-
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
- Directions
- Other Information
- Inactive ingredients
- Questions?
-
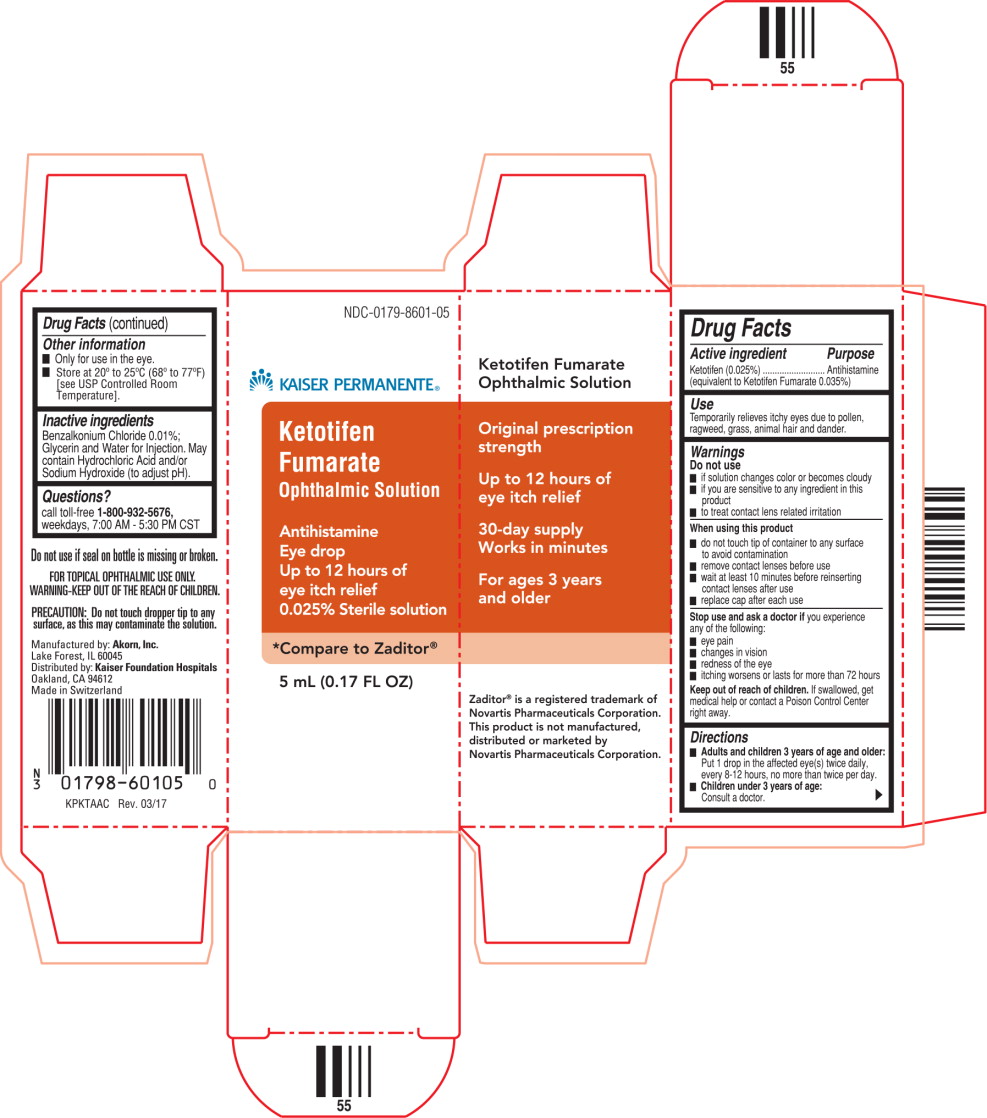
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 0179-8601-05
KAISER PERMANENTE ® Logo
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drop
5 mL (0.17 FL OZ) Sterile
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 0179-8601-05
KAISER PERMANENTE ® Logo
Ketotifen
Fumarate
Ophthalmic Solution
Antihistamine
Eye drop
Up to 12 hours of
eye itch relief
0.025% Sterile solution
*Compare to Zaditor ®
5 mL (0.17 FL OZ)
-
INGREDIENTS AND APPEARANCE
KETOTIFEN FUMARATE
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0179-8601 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOTIFEN FUMARATE (UNII: HBD503WORO) (KETOTIFEN - UNII:X49220T18G) KETOTIFEN 0.35 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0179-8601-05 1 in 1 CARTON 11/08/2011 11/01/2020 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077958 11/08/2011 11/01/2020 Labeler - KAISER FOUNDATION HOSPITALS (053052619) Establishment Name Address ID/FEI Business Operations Akorn AG 482198285 manufacture(0179-8601) , analysis(0179-8601) , pack(0179-8601) , label(0179-8601) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 603980319 manufacture(0179-8601) , repack(0179-8601) , analysis(0179-8601) , label(0179-8601) , pack(0179-8601) , relabel(0179-8601) , sterilize(0179-8601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.