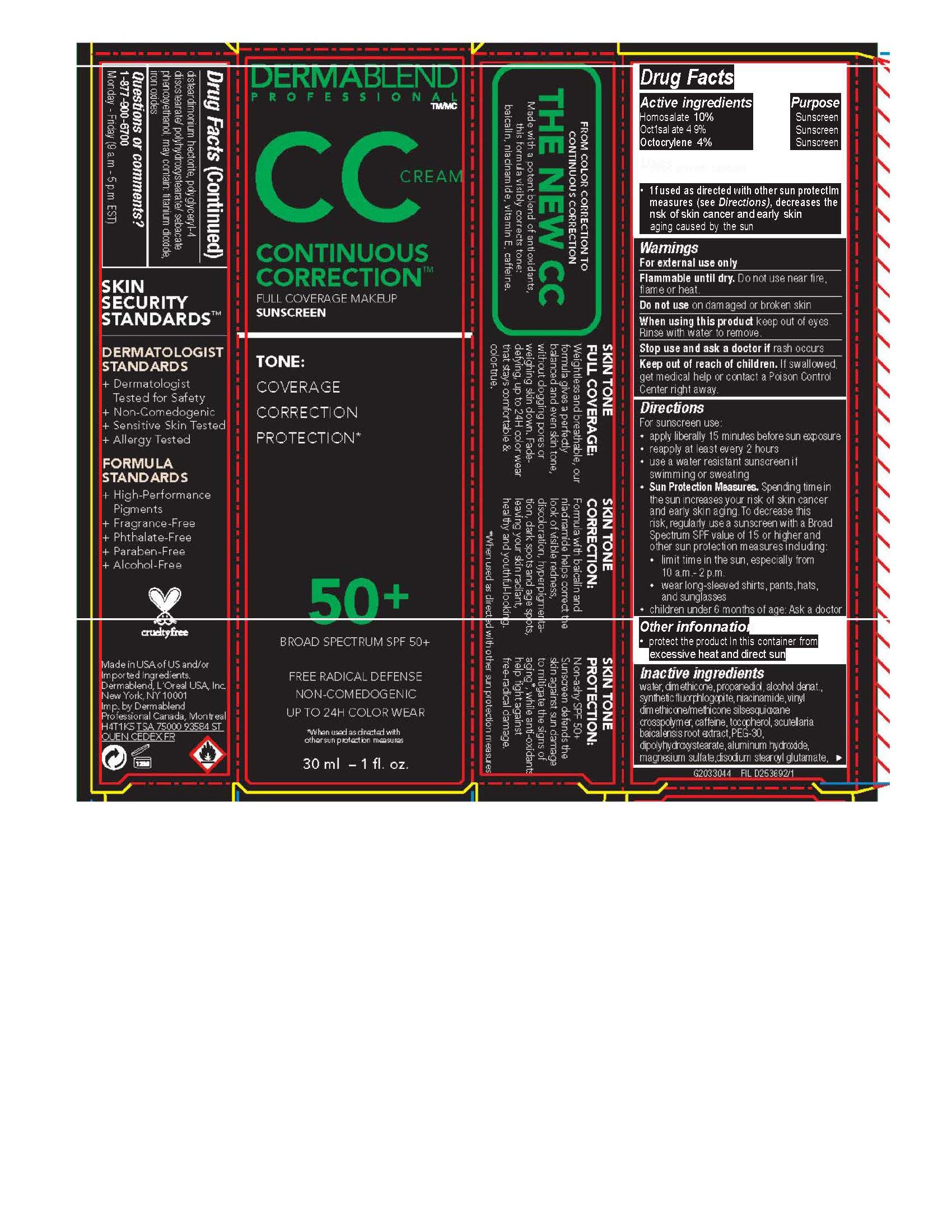
CONTINUOUS CORRECTION CREAM- homosalate 10% octisalate 4.9% octocrylene 4% cream
Continuous Correction Cream by
Drug Labeling and Warnings
Continuous Correction Cream by is a Otc medication manufactured, distributed, or labeled by Baxter of California, LLC, L'Oreal USA Products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use: ● apply liberally 15 minutes before sun exposure ● reapply at least every 2 hours ● use a water resistant sunscreen if swimming or sweating ● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: ● limit time in the sun, especially from 10 a.m. – 2 p.m. ● wear long-sleeved shirts, pants, hats, and sunglasses ● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, dimethicone, propanediol, alcohol denat., synthetic fluorphlogopite, niacinamide, vinyl dimethicone/methicone silsesquioxane crosspolymer, caffeine, tocopherol, scutellaria baicalensis root extract, PEG-30 dipolyhydroxystearate, aluminum hydroxide, magnesium sulfate, disodium stearoyl glutamate, disteardimonium hectorite, polyglyceryl-4 diisostearate/polyhydroxystearate/sebacate, phenoxyethanol, may contain: titanium dioxide, iron oxides
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CONTINUOUS CORRECTION CREAM
homosalate 10% octisalate 4.9% octocrylene 4% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 84203-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 40 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 49 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TOCOPHEROL (UNII: R0ZB2556P8) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) FERRIC OXIDE RED (UNII: 1K09F3G675) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) DIMETHICONE (UNII: 92RU3N3Y1O) PROPANEDIOL (UNII: 5965N8W85T) NIACINAMIDE (UNII: 25X51I8RD4) CAFFEINE (UNII: 3G6A5W338E) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84203-003-01 1 in 1 CARTON 03/29/2024 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/29/2024 Labeler - Baxter of California, LLC (031183172) Establishment Name Address ID/FEI Business Operations L'Oreal USA Products, Inc. 624244349 manufacture(84203-003)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.