Iristar Anti dandruff Shampoo. by Eubizrival LLC 83462-018 Complete
Iristar Anti dandruff Shampoo. by
Drug Labeling and Warnings
Iristar Anti dandruff Shampoo. by is a Otc medication manufactured, distributed, or labeled by Eubizrival LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
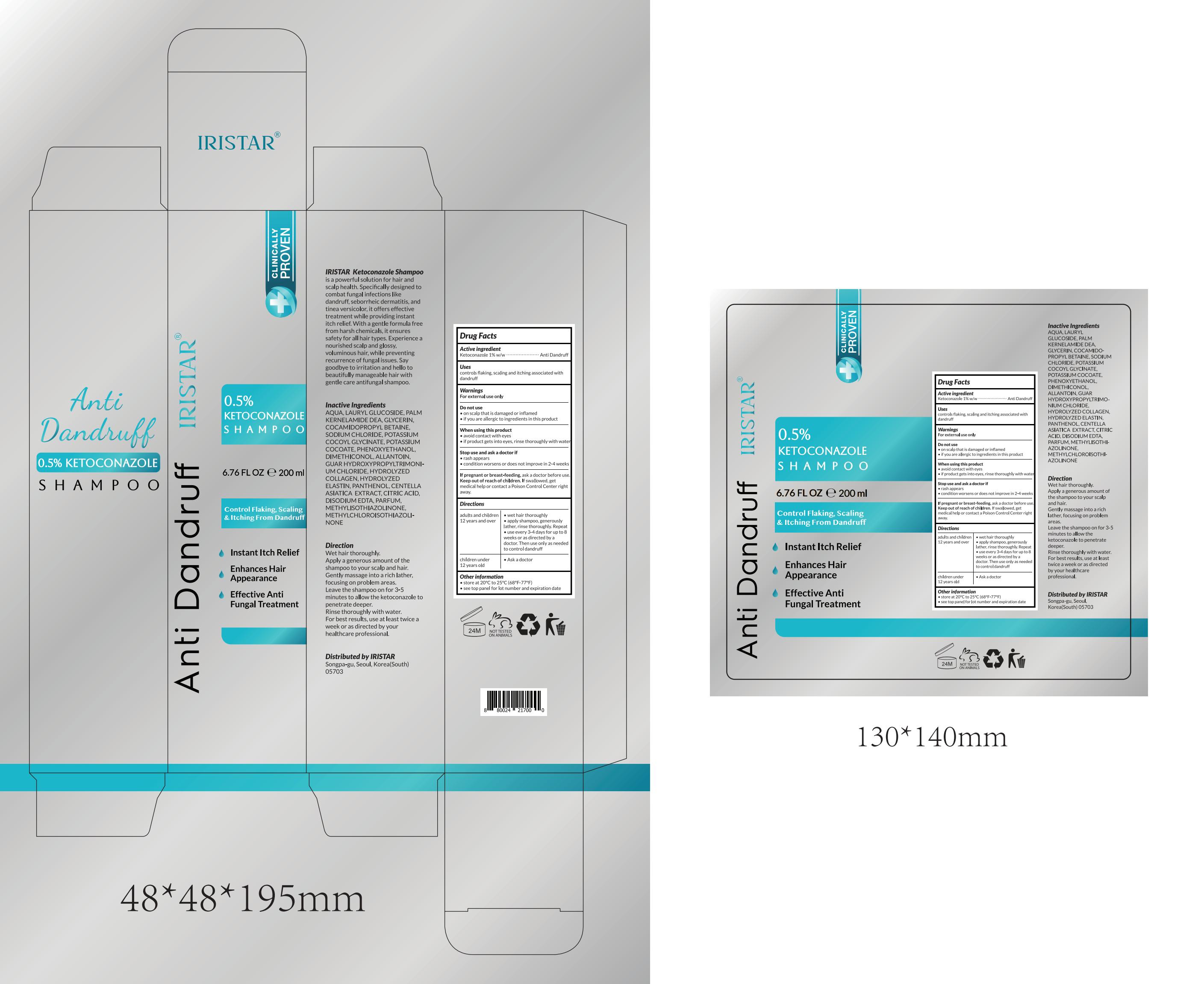
IRISTAR ANTI DANDRUFF SHAMPOO.- ketoconazole shampoo lotion/shampoo
Eubizrival LLC
----------
83462-018 Complete
Keep Oot Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children
12 years and over
wet hair thoroughly
apply shampoo, generously lather, rinse thoroughly. Repeat
use every 3-4 days for up to 8 weeks or as directed by a doctor. Then use only as needed to control dandruff
Children under 12 years old Ask a doctor
Other information
store at 20°C to 25°C (68°F-77°F)
see top panel for lot number and expiration date
Inactive ingredients
AQUA, LAURYL GLUCOSIDE, PALM KERNELAMIDE DEA, GLYCERIN, COCAMIDOPROPYL BETAINE, SODIUM CHLORIDE, POTASSIUM COCOYL GLYCINATE, POTASSIUM COCOATE, PHENOXYETHANOL, DIMETHICONOL, ALLANTOIN, GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, HYDROLYZED COLLAGEN, HYDROLYZED ELASTIN, PANTHENOL, CENTELLA ASIATICA EXTRACT, CITRIC ACID, DISODIUM EDTA, PARFUM, METHYLISOTHIAZOLINONE, METHYLCHLOROISOTHIAZOLINONE
| IRISTAR ANTI DANDRUFF SHAMPOO.
ketoconazole shampoo lotion/shampoo |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Eubizrival LLC (036572203) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eubizrival LLC | 036572203 | label(83462-018) , manufacture(83462-018) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.