These highlights do not include all the information needed to use DENAVIR® safely and effectively. See full prescribing information for DENAVIR®. DENAVIR® (penciclovir) Cream, for topical administrationInitial U.S. Approval: 1996
Denavir by
Drug Labeling and Warnings
Denavir by is a Prescription medication manufactured, distributed, or labeled by Prestium Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DENAVIR- penciclovir cream
Prestium Pharma, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DENAVIR® safely and effectively. See full prescribing information for DENAVIR®.
DENAVIR® (penciclovir) Cream, for topical administration Initial U.S. Approval: 1996 INDICATIONS AND USAGEDENAVIR® is a nucleoside analog HSV DNA polymerase inhibitor indicated for:
DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Prestium Pharma, Inc. at 1-866-897-5002 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSNo drug interaction studies have been performed with DENAVIR. Due to minimal systemic absorption of DENAVIR, systemic drug interactions are unlikely (7). USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 9/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
DENAVIR is a nucleoside analog HSV DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in adults and children 12 years of age or older.
2 DOSAGE AND ADMINISTRATION
DENAVIR should be applied every 2 hours during waking hours for a period of 4 days. Treatment should be started as early as possible (i.e., during the prodrome or when lesions appear).
3 DOSAGE FORMS AND STRENGTHS
Each gram of DENAVIR contains 10 mg of penciclovir in a cream base, which is equivalent to 1% (w/w).
4 CONTRAINDICATIONS
DENAVIR is contraindicated in patients with known hypersensitivity to the product or any of its components.
5 WARNINGS AND PRECAUTIONS
5.1 General
DENAVIR should only be used on herpes labialis on the lips and face. Because no data are available, application to human mucous membranes is not recommended. Particular care should be taken to avoid application in or near the eyes since it may cause irritation. Lesions that do not improve or that worsen on therapy should be evaluated for secondary bacterial infection. The effect of DENAVIR has not been established in immunocompromised patients.
6 ADVERSE REACTIONS
6.1 Clinical Studies
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In two double-blind, placebo-controlled trials, 1516 patients were treated with DENAVIR (penciclovir cream) and 1541 with placebo. One or more local adverse reactions were reported by 3% of the patients treated with DENAVIR and 4% of placebo-treated patients. The rates of reported local adverse reactions are shown in Table 1.
|
|
Penciclovir
|
Placebo
|
|
Applications site reaction |
1 |
2 |
Two studies, enrolling 108 healthy subjects, were conducted to evaluate the dermal tolerance of 5% penciclovir cream (a 5-fold higher concentration than the commercial formulation) compared to vehicle using repeated occluded patch testing methodology. The 5% penciclovir cream induced mild erythema in approximately one-half of the subjects exposed, an irritancy profile similar to the vehicle control in terms of severity and proportion of subjects with a response. No evidence of sensitization was observed.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of DENAVIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following events have been identified from worldwide post-marketing use of DENAVIR in treatment of recurrent herpes labialis (cold sores) in adults. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to DENAVIR.
General: Headache, oral/pharyngeal edema, parosmia.
Skin: Aggravated condition, decreased therapeutic response, local edema, pain, paresthesia, pruritus, skin discoloration, and urticaria.
7 DRUG INTERACTIONS
No drug interaction studies have been performed with DENAVIR. Due to minimal systemic absorption of DENAVIR, systemic drug interactions are unlikely.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Category B
There are no adequate and well-controlled studies in pregnant women.
Animal Data
No adverse effects on the course and outcome of pregnancy or on fetal development were noted in rats and rabbits following the intravenous administration of penciclovir at doses of 80 and 60 mg/kg/day, respectively (estimated human equivalent doses of 13 and 18 mg/kg/day for the rat and rabbit, respectively, based on body surface area conversion; the body surface area doses being 260 and 355x the maximum recommended dose following topical application of the penciclovir cream). Because animal reproduction studies are not always predictive of human response, penciclovir should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
There is no information on whether penciclovir is excreted in human milk after topical administration. However, following oral administration of famciclovir (the oral prodrug of penciclovir) to lactating rats, penciclovir was excreted in breast milk at concentrations higher than those seen in the plasma. Therefore, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother.
There are no data on the safety of penciclovir in newborns.
8.4 Pediatric Use
An open-label, uncontrolled trial with penciclovir cream 1% was conducted in 102 patients, ages 12-17 years, with recurrent herpes labialis. The frequency of adverse events was generally similar to the frequency previously reported for adult patients. Safety and effectiveness in pediatric patients less than 12 years of age have not been established.
10 OVERDOSAGE
Since penciclovir is poorly absorbed following oral administration, adverse reactions related to penciclovir ingestion are unlikely. There is no information on overdose.
11 DESCRIPTION
DENAVIR (penciclovir) cream 1% contains penciclovir, an antiviral agent active against herpes viruses. DENAVIR is available for topical administration as a 1% white cream. Each gram of DENAVIR contains 10 mg of penciclovir and the following inactive ingredients: cetostearyl alcohol, mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water and white petrolatum.
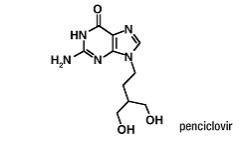
Chemically, penciclovir is known as 9-[4-hydroxy-3-(hydroxymethyl)butyl] guanine. Its molecular formula is C10H15N5O3; its molecular weight is 253.26. It is a synthetic acyclic guanine derivative and has the following structure:
Penciclovir is a white to pale yellow solid. At 20°C it has a solubility of 0.2 mg/mL in methanol, 1.3 mg/mL in propylene glycol, and 1.7 mg/mL in water. In aqueous buffer (pH 2) the solubility is 10.0 mg/mL. Penciclovir is not hygroscopic. Its partition coefficient in n-octanol/water at pH 7.5 is 0.024 (logP = -1.62).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Penciclovir is an antiviral agent active against herpes viruses [see Microbiology (12.4)].
12.3 Pharmacokinetics
Measurable penciclovir concentrations were not detected in plasma or urine of healthy male volunteers (n=12) following single or repeat application of the 1% cream at a dose of 180 mg penciclovir daily (approximately 67 times the estimated usual clinical dose).
Pediatric Patients: The systemic absorption of penciclovir following topical administration has not been evaluated in patients < 18 years of age.
12.4 Microbiology
Mechanism of Action: The antiviral compound penciclovir has inhibitory activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2). In cells infected with HSV-1 or HSV-2, the viral thymidine kinase phosphorylates penciclovir to a monophosphate form that, in turn, is converted by cellular kinases to the active form penciclovir triphosphate. Biochemical studies demonstrate that penciclovir triphosphate inhibits HSV polymerase competitively with deoxyguanosine triphosphate. Consequently, herpes viral DNA synthesis and, therefore, replication are selectively inhibited. Penciclovir triphosphate has an intracellular half-life of 10 hours in HSV-1 and 20 hours in HSV-2 infected cells grown in culture. However, the clinical significance of the intracellular half-life is unknown.
Antiviral Activity: In cell culture studies, penciclovir has antiviral activity against the following herpes viruses: HSV-1 and HSV-2. The antiviral activity of penciclovir against wild type strains grown on human foreskin fibroblasts was assessed with a plaque reduction assay and staining with crystal violet 3 days postinfection for HSV. The median EC50 values of penciclovir against laboratory and clinical isolates of HSV-1 and HSV-2 were 2 µM (range 1.2 to 2.4 µM, n=7) and 2.6 µM (range 1.6 to 11 µM, n=6), respectively.
Resistance: Penciclovir-resistant mutants of HSV can result from mutations in viral thymidine kinase (TK) and DNA polymerase genes. Mutations in the viral TK gene may lead to complete loss of TK activity (TK negative), reduced levels of TK activity (TK partial), or alteration in the ability of viral TK to phosphorylate the drug without an equivalent loss in the ability to phosphorylate thymidine (TK altered). The median EC50 values observed in a plaque reduction assays with penciclovir resistant HSV-1 and HSV-2 were 69 µM (range 14 to 115 µM, n=6) and 46 µM (range 4 to > 395 µM, n=9), respectively. The possibility of viral resistance to penciclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
Cross-resistance: Cross-resistance has been observed among HSV DNA polymerase inhibitors. The most commonly encountered acyclovir-resistant mutants that are deficient in viral thymidine kinase (TK negative) are also resistant to penciclovir.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In clinical trials, systemic drug exposure following topical administration of penciclovir cream was negligible, as the penciclovir content of all plasma and urine samples was below the limit of assay detection (0.1 mcg/mL and 10 mcg/mL, respectively). However, for the purpose of inter-species dose comparisons presented in the following sections, an assumption of 100% absorption of penciclovir from the topically applied product has been used. Based on the use of the maximal recommended topical dose of penciclovir of 0.05 mg/kg/day and an assumption of 100% absorption, the maximum theoretical plasma AUC0-24 hrs for penciclovir is approximately 0.129 mcg.hr/mL.
Carcinogenesis: Two-year carcinogenicity studies were conducted with famciclovir (the oral prodrug of penciclovir) in rats and mice. An increase in the incidence of mammary adenocarcinoma (a common tumor in female rats of the strain used) was seen in female rats receiving 600 mg/kg/day (approximately 395x the maximum theoretical human exposure to penciclovir following application of the topical product, based on area under the plasma concentration curve comparisons [24 hr. AUC]). No increases in tumor incidence were seen among male rats treated at doses up to 240 mg/kg/day (approximately 190x the maximum theoretical human AUC for penciclovir), or in male and female mice at doses up to 600 mg/kg/day (approximately 100x the maximum theoretical human AUC for penciclovir).
Mutagenesis: When tested in vitro, penciclovir did not cause an increase in gene mutation in the Ames assay using multiple strains of S. typhimurium or E. coli (at up to 20,000 mcg/plate), nor did it cause an increase in unscheduled DNA repair in mammalian HeLa S3 cells (at up to 5,000 mcg/mL). However, an increase in clastogenic responses was seen with penciclovir in the L5178Y mouse lymphoma cell assay (at doses ≥1000 mcg/mL) and, in human lymphocytes incubated in vitro at doses ≥250 mcg/mL. When tested in vivo, penciclovir caused an increase in micronuclei in mouse bone marrow following the intravenous administration of doses ≥500 mg/kg (≥810x the maximum human dose, based on body surface area conversion).
Impairment of Fertility: Testicular toxicity was observed in multiple animal species (rats and dogs) following repeated intravenous administration of penciclovir (160 mg/kg/day and 100 mg/kg/day, respectively, approximately 1155 and 3255x the maximum theoretical human AUC). Testicular changes seen in both species included atrophy of the seminiferous tubules and reductions in epididymal sperm counts and/or an increased incidence of sperm with abnormal morphology or reduced motility. Adverse testicular effects were related to an increasing dose or duration of exposure to penciclovir. No adverse testicular or reproductive effects (fertility and reproductive function) were observed in rats after 10 to 13 weeks dosing at 80 mg/kg/day, or testicular effects in dogs after 13 weeks dosing at 30 mg/kg/day (575 and 845x the maximum theoretical human AUC, respectively). Intravenously administered penciclovir had no effect on fertility or reproductive performance in female rats at doses of up to 80 mg/kg/day (260x the maximum human dose [BSA]).
There was no evidence of any clinically significant effects on sperm count, motility or morphology in 2 placebo-controlled clinical trials of Famvir® (famciclovir [the oral prodrug of penciclovir], 250 mg b.i.d.; n=66) in immunocompetent men with recurrent genital herpes, when dosing and follow-up were maintained for 18 and 8 weeks, respectively (approximately 2 and 1 spermatogenic cycles in the human).
14 CLINICAL STUDIES
DENAVIR was studied in two double-blind, placebo (vehicle)-controlled trials for the treatment of recurrent herpes labialis in which otherwise healthy adults were randomized to either DENAVIR or placebo. Therapy was to be initiated by the subjects within 1 hour of noticing signs or symptoms and continued for 4 days, with application of study medication every 2 hours while awake. In both studies, the mean duration of lesions was approximately one-half-day shorter in the subjects treated with DENAVIR (N=1,516) as compared to subjects treated with placebo (N=1,541) (approximately 4.5 days versus 5 days, respectively). The mean duration of lesion pain was also approximately one half-day shorter in the DENAVIR group compared to the placebo group.
16 HOW SUPPLIED/STORAGE AND HANDLING
DENAVIR is supplied in a 1.5 gram and 5 gram tube containing 10 mg of penciclovir per gram.
NDC: 50816-624-01
NDC 40076-624-05
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 General
DENAVIR is a prescription topical cream for the treatment of cold sores (recurrent herpes labialis) that occur on the face and lips. It is not a cure for cold sores and not all patients respond to it. Do not use if you are allergic to DENAVIR (penciclovir) or any of the ingredients in DENAVIR. Before you use DENAVIR, tell your doctor if you are pregnant, planning to become pregnant, or are breast-feeding.
17.2 Instructions for Use
Wash your hands. Your face should be clean and dry. Apply a layer of DENAVIR to cover only the cold sore area or the area of tingling (or other symptoms) before the cold sore appears. Rub in the cream until it disappears. Apply the cream every 2 hours during waking hours for 4 days. Treatment should be started at the earliest sign of a cold sore (i.e. tingling, redness, itching, or bump). Wash your hands with soap and water after using DENAVIR. Store DENAVIR at room temperature between 68°F to 77°F (20ºC to 25ºC). Keep out of reach of children.
17.3 Possible Side Effects
DENAVIR was well tolerated in clinical studies in patients with cold sores. Common skin-related side effects that occurred when DENAVIR was applied are application site reactions, local anesthesia, and rash. Taste perversion was also reported.
September 2013
Manufactured for Prestium Pharma, Inc.
Newtown, PA 18940
by Novartis Pharma GmbH, Wehr, Germany
Denavir® is licensed to Prestium Pharma, Inc. from Denco Asset, LLC.
©2013 Prestium Pharma, Inc.
PRINCIPAL DISPLAY PANEL – 1%
NDC: 40076-624-05
Denavir®
(penciclovir cream) 1%
5 g (Net Wt.)
For Topical Use Only
| DENAVIR
penciclovir cream |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Prestium Pharma, Inc. (078304674) |