Celecoxib by Alembic Pharmaceuticals Limited CELECOXIB capsule
Celecoxib by
Drug Labeling and Warnings
Celecoxib by is a Prescription medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use celecoxib safely and effectively. See full prescribing information for Celecoxib.
CELECOXIB capsules, for oral use
Initial U.S. Approval: 1998WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS
See full prescribing information for complete boxed warning
CardiovascularRisk
- Celecoxib may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs may have a similar risk. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (5.1, 14.6)
- Celecoxib is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery. (4, 5.1)
Gastrointestinal Risk
NSAIDs, including celecoxib, cause an increased risk of serious gastro intestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and with out warning symptoms. Elderly patients are at greater risk for serious gastro intestinal (GI) events. (5.4)INDICATIONS AND USAGE
Celecoxib is a non steroidal anti-inflammatory drug indicated for: (1)
- Osteoarthritis (OA) (1.1)
- Rheumatoid Arthritis (RA) (1.2)
- Juvenile Rheumatoid Arthritis (JRA) in patients 2 years and older(1.3)
- Ankylosing Spondylitis (AS) (1.4)
- Acute Pain (AP) (1.5)
- Primary Dysmenorrhea (PD) (1.6)
DOSAGE AND ADMINISTRATION
Use lowest effective dose for the shortest duration consistent with treatment goals for the individual patient. (1,5.1,5.4) (2)
- OA : 200 mg once daily or 100 mg twice daily (2.1,14.1)
- RA : 100 to 200 mg twice daily (2.2,14.2)
- JRA : 50 mg twice daily in patients 10 to 25 kg. 100 mg twice daily in patients more than 25 kg (2.3,14.3)
- AS : 200 mg once daily single dose or 100mg twice daily. If no effect is observed after 6 weeks, a trial of 400 mg (single or divided doses) may be of benefit (2.4,14.4)
- AP and PD : 400 mg initially, followed by 200 mg dose if needed on first day. On subsequent days, 200 mg twice daily as needed (2.5,14.5)
Reduce daily dose by 50% inpatients with moderate hepatic impairment (Child-PughClassB). (2)
Consider a dose reduction by 50% (oral ternative management for JRA) in patients who are know nor suspected to be CYP2C9 poor metabolizers, (2.6,8.4,8.8,12.3). (2)
DOSAGE FORMS AND STRENGTHS
Capsules: 50 mg, 100 mg, 200 mg and 400 mg (3) (3)
CONTRAINDICATIONS
- Known hypersensitivity to celecoxib or sulfonamides (4)
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4, 5.7, 5.8, 5.13)
- Use during the perioperative period in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1)
WARNINGS AND PRECAUTIONS
- Serious and potentially fatal cardiovascular (CV) thrombotic events, myocardial infarction, and stroke. Patients with known CV disease/risk factors may be at greater risk (5.1, 14.6, 17.2).
- Serious gastrointestinal (GI) adverse events, which can be fatal. The risk is greater in patients with a prior history of ulcer disease or GI bleeding, and in patients at high risk for GI events, especially the elderly. Celecoxib should be used with caution in these patients (5.4, 8.5, 14.6, 17.3).
- Elevated liver enzymes and, rarely, severe hepatic reactions. Discontinue use of celecoxib immediately if abnormal liver enzymes persist or worsen (5.5, 17.4).
- New onset or worsening of hypertension. Blood pressure should be monitored closely during treatment with celecoxib (5.2, 7.4, 17.2).
- Fluid retention and edema. Celecoxib should be used with caution in patients with fluid retention or heart failure (5.3, 17.6).
- Renal papillary necrosis and other renal injury with long term use. Use celecoxib with caution in the elderly, those with impaired renal function, heart failure, liver dysfunction, and those taking diuretics, ACE-inhibitors, or angiotensin II antagonists (5.6, 7.4, 8.7, 17.6).
- Anaphylactoid reactions. Do not use celecoxib in patients with the aspirin triad (5.7, 10, 17.7).
- Serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal and can occur without warning even without known prior sulfa allergy. Discontinue celecoxib at first appearance of rash or skin reactions (5.8, 17.5).
ADVERSE REACTIONS
Most common adverse reactions in arthritis trials (>2% and >placebo): abdominal pain, diarrhea, dyspepsia, flatulence, peripheral edema, accidental injury, dizziness, pharyngitis, rhinitis, sinusitis, upper respiratory tract infection, rash (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
Concomitant use of celecoxib and warfarin may result in increased risk of bleeding complications. (7.1)
Concomitant use of celecoxib increases lithium plasma levels. (7.2)
Concomitant use of celecoxib may reduce the antihypertensive effect of ACE Inhibitors and angiotensin II antagonists (7.4)
Use caution with drugs known to inhibit P450 2C9 or metabolized by 2D6 due to the potential for increased plasma levels (2.6, 8.4, 8.8, 12.3) (7)USE IN SPECIFIC POPULATIONS
- Pregnancy Category C prior to 30 weeks gestation; Category D starting at 30 weeks gestation (5.9, 8.1, 17.8)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS
1 INDICATIONS AND USAGE
1.1 Osteoarthritis (OA)
1.2 Rheumatoid Arthritis (RA)
1.3 Juvenile Rheumatoid Arthritis (JRA)
1.4 Ankylosing Spondylitis (AS)
1.5 Acute Pain (AP)
1.6 Primary Dysmenorrhea (PD)
2 DOSAGE AND ADMINISTRATION
2.1 Osteoarthritis
2.2 Rheumatoid Arthritis
2.3 Juvenile Rheumatoid Arthritis
2.4 Ankylosing Spondylitis
2.5 Management of Acute Pain and Treatment of Primary Dysmenorrhea
2.6 Special Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
5.2 Hypertension
5.3 Congestive Heart Failure and Edema
5.4 Gastrointestinal (GI) Effects Risk of GI Ulceration, Bleeding, and Perforation
5.5 Hepatic Effects
5.6 Renal Effects
5.7 Anaphylactoid Reactions
5.8 Skin Reactions
5.9 Pregnancy
5.10 Corticosteroid Treatment
5.11 Hematological Effects
5.12 Disseminated Intravascular Coagulation (DIC)
5.13 Preexisting Asthma
5.14 Laboratory Tests
5.15 Inflammation
5.16 Concomitant NSAID Use
6 ADVERSE REACTIONS
6.1 Pre-marketing Controlled Arthritis Trials
6.2 The Celecoxib Long-Term Arthritis Safety Study
6.3 Juvenile Rheumatoid Arthritis Study
6.4 Other Pre-Approval Studies
6.5 The APC and PreSAP Trials
7 DRUG INTERACTIONS
7.1 Warfarin
7.2 Lithium
7.3 Aspirin
7.4 ACE-inhibitors and Angiotensin II Antagonists
7.5 Fluconazole
7.6 Furosemide
7.7 Methotrexate
7.8 Concomitant NSAID Use
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Insufficiency
8.7 Renal Insufficiency
8.8 Poor Metabolizers of CYP2C9 Substrates
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
14.1 Osteoarthritis
14.2 Rheumatoid Arthritis
14.3 Juvenile Rheumatoid Arthritis
14.4 Ankylosing Spondylitis
14.5 Analgesia, including Primary Dysmenorrhea
14.6 Special Studies
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Medication Guide
17.2 Cardiovascular Effects
17.3 Gastrointestinal Effects
17.4 Hepatic Effects
17.5 Adverse Skin Reactions
17.6 Weight Gain and Edema
17.7 Anaphylactoid Reactions
17.8 Effects During Pregnancy
17.9 Preexisting Asthma
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS
Cardiovascular Risk
- Celecoxib may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. All nonsteroidal anti-inflammatory drugs (NSAIDs) may have a similar risk. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (5.1, 14.6).
- Celecoxib is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1).
Gastrointestinal Risk
NSAIDs, including celecoxib, cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (5.4).
-
1 INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of celecoxib and other treatment options before deciding to use celecoxib. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)]
1.1 Osteoarthritis (OA)
Celecoxib is indicated for relief of the signs and symptoms of OA [see Clinical Studies (14.1)]
1.2 Rheumatoid Arthritis (RA)
Celecoxib is indicated for relief of the signs and symptoms of RA [see Clinical Studies (14.2)]
1.3 Juvenile Rheumatoid Arthritis (JRA)
Celecoxib is indicated for relief of the signs and symptoms of JRA in patients 2 years and older [see Clinical Studies (14.3)]
1.4 Ankylosing Spondylitis (AS)
Celecoxib is indicated for the relief of signs and symptoms of AS [see Clinical Studies (14.4)]
-
2 DOSAGE AND ADMINISTRATION
Use lowest effective dose for the shortest duration consistent with treatment goals for the individual patient. These doses can be given without regard to timing of meals.
2.1 Osteoarthritis
For relief of the signs and symptoms of OA the recommended oral dose is 200 mg per day administered as a single dose or as 100 mg twice daily.
2.2 Rheumatoid Arthritis
For relief of the signs and symptoms of RA the recommended oral dose is 100 to 200 mg twice daily.
2.3 Juvenile Rheumatoid Arthritis
For the relief of the signs and symptoms of JRA the recommended oral dose for pediatric patients (age 2 years and older) is based on weight. For patients ≥10 kg to ≤25 kg the recommended dose is 50 mg twice daily. For patients >25 kg the recommended dose is 100 mg twice daily.
For patients who have difficulty swallowing capsules, the contents of a celecoxib capsule can be added to applesauce. The entire capsule contents are carefully emptied onto a level teaspoon of cool or room temperature applesauce and ingested immediately with water. The sprinkled capsule contents on applesauce are stable for up to 6 hours under refrigerated conditions (2 to 8°C/ 35 to 45°F).
2.4 Ankylosing Spondylitis
For the management of the signs and symptoms of AS, the recommended dose of celecoxibis 200 mg daily in single (once per day) or divided (twice per day) doses. If no effect is observed after 6 weeks, a trial of 400 mg daily may be worthwhile. If no effect is observed after 6 weeks on 400 mg daily, a response is not likely and consideration should be given to alternate treatment options.
2.5 Management of Acute Pain and Treatment of Primary Dysmenorrhea
The recommended dose of celecoxib is 400 mg initially, followed by an additional 200 mg dose if needed on the first day. On subsequent days, the recommended dose is 200 mg twice daily as needed.
2.6 Special Populations
Hepatic insufficiency: The daily recommended dose of celecoxib in patients with moderate hepatic impairment (Child-Pugh Class B) should be reduced by 50%. The use of celecoxib in patients with severe hepatic impairment is not recommended [see Warnings and Precautions (5.5),Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Poor Metabolizers of CYP2C9 Substrates: Patients who are known or suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin, phenytoin) should be administered celecoxib with caution. Consider starting treatment at half the lowest recommended dose in poor metabolizers (i.e. CYP2C9*3/*3). Consider using alternative management in JRA patients who are poor metabolizes [see Use in Specific populations (8.8) and Clinical Pharmacology (12.5)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Celecoxib is contraindicated:
- In patients with known hypersensitivity to celecoxib, aspirin, or other NSAIDs.
- In patients who have demonstrated allergic-type reactions to sulfonamides.
- In patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe anaphylactoid reactions to NSAIDs, some of them fatal, have been reported in such patients [see Warnings and Precautions (5.7,5.13)].
- For the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG)surgery [see Warnings and Precautions(5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
Chronic use of celecoxib may cause an increased risk of serious adverse cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. In the APC (Adenoma Prevention with Celecoxib) trial, the hazard ratio for the composite endpoint of cardiovascular death, MI, or stroke was 3.4 (95% CI 1.4 to 8.5) for celecoxib 400 mg twice daily and 2.8 (95% CI 1.1 to 7.2) with celecoxib 200 mg twice daily compared to placebo. Cumulative rates for this composite endpoint over 3 years were 3% (20/671 subjects) and 2.5% (17/685 subjects), respectively, compared to 0.9% (6/679 subjects) with placebo treatment. The increases in both celecoxib dose groups versus placebo-treated patients were mainly due to an increased incidence of myocardial infarction [see Clinical Studies (14.6)].
All NSAIDs, both COX-2 selective and non-selective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with celecoxib, the lowest effective dose should be used for the shortest duration consistent with individual patient treatment goals. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV toxicity and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and celecoxib does increase the risk of serious GI events [see Warnings and Precautions (5.4)].
Two large, controlled, clinical trials of a different COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke [see Contraindications (4)].
5.2 Hypertension
As with all NSAIDs, celecoxib can lead to the onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including celecoxib, should be used with caution in patients with hypertension. Blood pressure should be monitored closely during the initiation of therapy with celecoxib and throughout the course of therapy. The rates of hypertension from the CLASS trial in the celecoxib, ibuprofen and diclofenac-treated patients were 2.4%, 4.2% and 2.5%, respectively [see Clinical Studies (14.6)].
5.3 Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs, including celecoxib [see Adverse Reactions (6.1)]. In the CLASS study [see Clinical Studies (14.6)], the Kaplan-Meier cumulative rates at 9 months of peripheral edema in patients on celecoxib 400 mg twice daily (4-fold and 2-fold the recommended OA and RA doses, respectively), ibuprofen 800 mg three times daily and diclofenac 75 mg twice daily were 4.5%, 6.9% and 4.7%, respectively. Celecoxib should be used with caution in patients with fluid retention or heart failure.
5.4 Gastrointestinal (GI) Effects Risk of GI Ulceration, Bleeding, and Perforation
NSAIDs, including celecoxib, can cause serious gastrointestinal events including bleeding, ulceration, and perforation of the stomach, small intestine or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Complicated and symptomatic ulcer rates were 0.78% at nine months for all patients in the CLASS trial, and 2.19% for the subgroup on low-dose ASA. Patients 65 years of age and older had an incidence of 1.40% at nine months, 3.06% when also taking ASA [see Clinical Studies (14.6)]. With longer duration of use of NSAIDs, there is a trend for increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in patients with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status.Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event, the lowest effective dose should be used for the shortest duration consistent with individual patient treatment goals. Physicians and patients should remain alert for signs and symptoms of GI ulceration and bleeding during celecoxib therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.
5.5 Hepatic Effects
Border line elevations of one or more liver-associated enzymes may occur in up to 15% of patients taking NSAIDs, and notable elevations of ALT or AST (approximately 3 or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure (some with fatal outcome) have been reported with NSAIDs, including celecoxib [see Adverse Reactions (6.1)]. In controlled clinical trials of celecoxib, the incidence of borderline elevations (greater than or equal to 1.2 times and less than 3 times the upper limit of normal) of liver associated enzymes was 6% for celecoxib and 5% for placebo, and approximately 0.2% of patients taking celecoxib and 0.3% of patients taking placebo had notable elevations of ALT and AST.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be monitored carefully for evidence of the development of a more severe hepatic reaction while on therapy with celecoxib. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), celecoxib should be discontinued.
5.6 Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics, ACE-inhibitors, angiotensin II receptor antagonists, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state. Clinical trials with celecoxib have shown renal effects similar to those observed with comparator NSAIDs.
No information is available from controlled clinical studies regarding the use of celecoxib in patients with advanced renal disease. Therefore, treatment with celecoxib is not recommended in these patients with advanced renal disease. If celecoxib therapy must be initiated, close monitoring of the patient’s renal function is advisable.
5.7 Anaphylactoid Reactions
As with NSAIDs in general, anaphylactoid reactions have occurred in patients without known prior exposure to celecoxib. In post-marketing experience, rare cases of anaphylactic reactions and angioedema have been reported in patients receiving celecoxib. Celecoxib should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs [see Contraindications (4), Warnings and Precautions (5.7)]. Emergency help should be sought in cases where an anaphylactoid reaction occurs.
5.8 Skin Reactions
Celecoxib is a sulfonamide and can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events can occur without warning and in patients without prior known sulfa allergy. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
5.9 Pregnancy
In late pregnancy, starting at 30 weeks gestation, celecoxib should be avoided because it may cause premature closure of the ductus arteriosus [see Use in Specific Populations (8.1)].
5.10 Corticosteroid Treatment
Celecoxib cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to exacerbation of corticosteroid-responsive illness. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
5.11 Hematological Effects
Anemia is sometimes seen in patients receiving celecoxib. In controlled clinical trials the incidence of anemia was 0.6% with celecoxib and 0.4% with placebo. Patients on long-term treatment with celecoxib should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia or blood loss. Celecoxib does not generally affect platelet counts, prothrombin time (PT), or partial thromboplastin time (PTT), and does not inhibit platelet aggregation at indicated dosages [see Clinical Pharmacology (12.2)].
5.12 Disseminated Intravascular Coagulation (DIC)
Celecoxib should be used only with caution in pediatric patients with systemic onset JRA due to the risk of disseminated intravascular coagulation.
5.13 Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, celecoxib should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
5.14 Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have a CBC and a chemistry profile checked periodically. If abnormal liver tests or renal tests persist or worsen, celecoxib should be discontinued.
In controlled clinical trials, elevated BUN occurred more frequently in patients receiving celecoxib compared with patients on placebo. This laboratory abnormality was also seen in patients who received comparator NSAIDs in these studies. The clinical significance of this abnormality has not been established.
-
6 ADVERSE REACTIONS
Of the celecoxib-treated patients in the pre-marketing controlled clinical trials, approximately 4,250 were patients with OA, approximately 2,100 were patients with RA, and approximately 1,050 were patients with post-surgical pain. More than 8,500 patients received a total daily dose of celecoxib of 200 mg (100 mg twice daily or 200 mg once daily) or more, including more than 400 treated at 800 mg (400 mg twice daily). Approximately 3,900 patients received celecoxib at these doses for 6 months or more; approximately 2,300 of these have received it for 1 year or more and 124 of these have received it for 2 years or more.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
6.1 Pre-marketing Controlled Arthritis Trials
Table 1 lists all adverse events, regardless of causality, occurring in ≥2% of patients receiving celecoxibfrom 12 controlled studies conducted in patients with OA or RA that included a placebo and/or a positive control group. Since these 12 trials were of different durations, and patients in the trials may not have been exposed for the same duration of time, these percentages do not capture cumulative rates of occurrence.
Table 1: Adverse Events Occurring in >2% of Celecoxib Patients from Pre-marketing Controlled Arthritis Trials
CBX
N=4,146
Placebo
N=1,864
NAP
N=1,366
DCF
N=387
IBU
N=345
Gastrointestinal
AbdominalPain Diarrhea
Dyspepsia
Flatulence
Nausea
4.1% 5.6% 8.8% 2.2% 3.5%
2.8% 3.8% 6.2% 1% 4.2%
7.7% 5.3% 12.2%3.6% 6%
9% 9.3% 10.9%4.1% 3.4%
9% 5.8% 12.8% 3.5% 6.7%
Bodyasawhole
BackPain PeripheralEdema Injury-Accidental
2.8% 2.1% 2.9%
3.6% 1.1% 2.3%
2.2% 2.1% 3%
2.6% 1% 2.6%
0.9% 3.5% 3.2%
Central, Peripheral Nervoussystem
Dizziness Headache
2%15.8%
1.7% 20.2%
2.6% 14.5%
1.3% 15.5%
2.3% 15.4%
PsychiatricInsomnia
2.3%
2.3%
2.9%
1.3%
1.4%
Respiratory Pharyngitis Rhinitis Sinusitis UpperRespiratory Infection
2.3% 2%
5% 8.1%
1.1% 1.3% 4.3% 6.7%
1.7% 2.4% 4% 9.9%
1.6% 2.3% 5.4% 9.8%
2.6% 0.6% 5.8% 9.9%
Skin
Rash
2.2%
2.1%
2.1%
1.3%
1.2%
CBX = Celecoxib 100 to 200 mg twice daily or 200 mg once daily;
NAP = Naproxen 500 mg twice daily;
DCF = Diclofenac 75 mg twice daily;
IBU = Ibuprofen 800 mg three times daily.
In placebo- or active-controlled clinical trials, the discontinuation rate due to adverse events was 7.1% for patients receiving celecoxib and 6.1% for patients receiving placebo. Among the most common reasons for discontinuation due to adverse events in the celecoxib treatment groups were dyspepsia and abdominal pain (cited as reasons for discontinuation in 0.8% and 0.7% of celecoxib patients, respectively). Among patients receiving placebo, 0.6% discontinued due to dyspepsia and 0.6% withdrew due to abdominal pain.
The following adverse reactions occurred in 0.1 to 1.9% of patients treated with celecoxib (100 to 200 mg twice daily or 200 mg once daily):
Gastrointestinal:
Constipation, diverticulitis, dysphagia, eructation, esophagitis, gastritis, gastroenteritis, gastroesophageal reflux, hemorrhoids, hiatal hernia, melena, dry mouth, stomatitis, tenesmus, vomiting
Cardiovascular:
Aggravated hypertension, angina pectoris, coronary artery disorder, myocardial infarction
General:
Allergy aggravated, allergic reaction, chest pain, cyst NOS, edema generalized, face edema, fatigue, fever, hot flushes, influenza-like symptoms, pain, peripheral pain.
Central, peripheral nervous system:
Leg cramps, hypertonia, hypoesthesia, migraine, paresthesia, vertigo
Hearing and vestibular:
Deafness, tinnitus
Heart rate and rhythm:
Palpitation, tachycardia
Liver and biliary:
Hepatic function abnormal, SGOT increased, SGPT increased
Metabolic and nutritional:
BUN increased, CPK increased, hypercholesterolemia, hyperglycemia, hypokalemia, NPN increased, creatinine increased, alkaline phosphatase increased, weight increased
Musculoskeletal:
Arthralgia, arthrosis, myalgia, synovitis, tendinitis
Platelets (bleedingor clotting):
Ecchymosis, epistaxis, thrombocythemia,
Psychiatric:
Anorexia, anxiety, appetite increased, depression, nervousness, somnolence
Hemic:
Anemia
Respiratory:
Bronchitis, bronchospasm, bronchospasm aggravated, coughing, dyspnea, laryngitis, pneumonia
Skin and appendages:
Alopecia, dermatitis, photosensitivity reaction, pruritus, rash erythematous, rash maculopapular, skin disorder, skin dry, sweating increased, urticaria
Application site disorders:
Cellulitis, dermatitis contact
Urinary:
Albuminuria, cystitis, dysuria, hematuria, micturition frequency, renal calculus
The following serious adverse events (causality not evaluated) occurred in <0.1% of patients (cases reported only in post-marketing experience are indicated in italics):
Cardiovascular:
Syncope, congestive heart failure, ventricular fibrillation, pulmonary embolism, cerebrovascular accident, peripheral gangrene, thrombophlebitis, vasculitis, deep venous thrombosis
Gastrointestinal:
Intestinal obstruction, intestinal perforation, gastrointestinal bleeding, colitis with bleeding, esophageal perforation, pancreatitis, ileus
Liver and biliary:
Cholelithiasis, hepatitis, jaundice, liver failure
Hemic and lymphatic:
Thrombocytopenia, agranulocytosis, aplastic anemia, pancytopenia, leucopenia
Metabolic:
Hypoglycemia, hyponatremia
Nervous:
Ataxia, suicide, aseptic meningitis, ageusia, anosmia, fatal intracranial hemorrhage [see Drug Interactions (7.1)]
Renal:
Acute renal failure, interstitial nephritis
Skin:
Erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis
General:
Sepsis, sudden death, anaphylactoid reaction, angioedema
6.2 The Celecoxib Long-Term Arthritis Safety Study
[seeSpecial Studies (14.6)]
Hematological Events: The incidence of clinically significant decreases in hemoglobin (>2 g/dL) was lower in patients on celecoxib 400 mg twice daily (0.5%) compared to patients on either diclofenac 75 mg twice daily (1.3%) or ibuprofen 800 mg three times daily 1.9%. The lower incidence of events with celecoxib was maintained with or without ASA use [see Clinical Pharmacology (12.2)].
Withdrawals/Serious Adverse Events: Kaplan-Meier cumulative rates at 9 months for withdrawals due to adverse events for celecoxib, diclofenac and ibuprofen were 24%, 29%, and 26%, respectively. Rates for serious adverse events (i.e., causing hospitalization or felt to be life-threatening or otherwise medically significant), regardless of causality, were not different across treatment groups (8%, 7%, and 8%, respectively).
6.3 Juvenile Rheumatoid Arthritis Study
In a 12-week, double-blind, active-controlled study, 242 JRA patients 2 years to 17 years of age were treated with celecoxib or naproxen; 77 JRA patients were treated with celecoxib 3 mg/kg BID, 82 patients were treated with celecoxib 6 mg/kg BID, and 83 patients were treated with naproxen 7.5 mg/kg BID. The most commonly occurring (≥5%) adverse events in celecoxib treated patients were headache, fever (pyrexia), upper abdominal pain, cough, nasopharyngitis, abdominal pain, nausea, arthralgia, diarrhea and vomiting. The most commonly occurring (≥5%) adverse experiences for naproxen-treated patients were headache, nausea, vomiting, fever, upper abdominal pain, diarrhea, cough, abdominal pain, and dizziness (Table 2). Compared with naproxen, celecoxib at doses of 3 and 6 mg/kg BID had no observable deleterious effect on growth and development during the course of the 12-week double-blind study. There was no substantial difference in the number of clinical exacerbations of uveitis or systemic features of JRA among treatment groups.
In a 12-week, open-label extension of the double-blind study described above, 202 JRA patients were treated with celecoxib 6 mg/kg BID. The incidence of adverse events was similar to that observed during the double-blind study; no unexpected adverse events of clinical importance emerged.
Table 2: Adverse Events Occurring in ≥5% of JRA Patients in Any Treatment Group, by System Organ Class (% of patients with events)
System Organ Class
Preferred Term
All Doses Twice Daily
Celecoxib 3 mg/kg
N=77
Celecoxib 6 mg/kg
N=82
Naproxen 7.5 mg/kg
N=83
Any Event
64
70
72
Eye Disorders
5
5
5
Gastrointestinal
26
24
36
AbdominalpainNOS
Abdominalpainupper
Vomiting NOS
DiarrheaNOS
Nausea
4
8
3
5
7
7
6
6
4
4
7
10
11
8
11
General
13
11
18
Pyrexia
8
9
11
Infections
25
20
27
Nasopharyngitis
5
6
5
Injury and Poisoning
4
6
5
Investigations*
3
11
7
Musculoskeletal
8
10
17
Arthralgia
3
7
4
Nervous System
17
11
21
Headache NOS
Dizziness (excl vertigo)
13
1
10
1
16
7
Respiratory
8
15
15
Cough
7
7
8
Skin & Subcutaneous
10
7
18
* Abnormal laboratory tests, which include: Prolonged activated partial thromboplastin time, Bacteriuria NOS present, Blood creatine phosphokinase increased, Blood culture positive, Blood glucose increased, Blood pressure increased, Blood uric acid increased, Hematocrit decreased, Hematuria present, Hemoglobin decreased, Liver function tests NOS abnormal, Proteinuria present, Transaminase NOS increased, Urine analysis abnormal NOS
6.4 Other Pre-Approval Studies
Adverse Events from Ankylosing Spondylitis Studies: A total of 378 patients were treated with celecoxib in placebo- and active-controlled AS studies. Doses up to 400 mg once daily were studied. The types of adverse events reported in the AS studies were similar to those reported in the OA/RA studies.
Adverse Events from Analgesia and Dysmenorrhea Studies: Approximately 1,700 patients were treated with celecoxib in analgesia and dysmenorrhea studies. All patients in post-oral surgery pain studies received a single dose of study medication. Doses up to 600 mg/day of celecoxib were studied in primary dysmenorrhea and post-orthopedic surgery pain studies. The types of adverse events in the analgesia and dysmenorrhea studies were similar to those reported in arthritis studies. The only additional adverse event reported was post-dental extraction alveolar osteitis (dry socket) in the post-oral surgery pain studies.
6.5 The APC and PreSAP Trials
Adverse reactions from long-term, placebo-controlled polyp prevention studies:Exposure to celecoxib in the APC and PreSAP trials was 400 to 800 mg daily for up to 3 years [see Special Studies Adenomatous Polyp Prevention Studies (14.6)].
Some adverse reactions occurred in higher percentages of patients than in the arthritis pre-marketing trials (treatment durations up to 12 weeks; see adverse events from celecoxib pre-marketing controlled arthritis trials, above). The adverse reactions for which these differences in patients treated with celecoxibwere greater as compared to the arthritis pre-marketing trials were as follows:
Celecoxib
(400 to 800 mg daily)N=2,285
Placebo
N = 1,303
Diarrhea
10.5%
7%
Gastroesophageal reflux disease
4.7%
3.1%
Nausea
6.8%
5.3%
Vomiting
3.2%
2.1%
Dyspnea
2.8%
1.6%
Hypertension
12.5%
9.8%
The following additional adverse reactions occurred in ≥0.1% and <1% of patients taking celecoxib, at an incidence greater than placebo in the long-term polyp prevention studies, and were either not reported during the controlled arthritis pre-marketing trials or occurred with greater frequency in the long-term, placebo-controlled polyp prevention studies:
Nervous system disorders: Cerebral infarction
Eyedisorders: Vitreous floaters, conjunctival hemorrhage
Ear and labyrinth: Labyrinthitis
Cardiac disorders: Angina unstable, aortic valve incompetence, coronary artery atherosclerosis, sinus bradycardia, ventricular hypertrophy
Vascular disorders: Deep vein thrombosis
Reproductive system and breast disorders: Ovarian cyst
Investigations: Blood potassium increased,blood sodium increased, blood testosterone decreased
Injury, poisoning and procedural complications: Epicondylitis, tendonrupture -
7 DRUG INTERACTIONS
General: Celecoxib metabolism is predominantly mediated via cytochrome P450(CYP)2C9 in the liver. Co-administration of celecoxib with drugs that are known to inhibit CYP2C9 should be done with caution. Significant interactions may occur when celecoxib is administered together with drugs that inhibit CYP2C9. In vitro studies indicate that celecoxib, although not a substrate, is an inhibitor of CYP2D6. Therefore, there is a potential for an in vivo drug interaction with drugs that are metabolized by CYP2D6.
7.1 Warfarin
Anticoagulant activity should be monitored, particularly in the first few days, after initiating or changing celecoxib therapy in patients receiving warfarin or similar agents, since these patients are at an increased risk of bleeding complications. The effect of celecoxib on the anticoagulant effect of warfarin was studied in a group of healthy subjects receiving daily 2 to 5 mg doses of warfarin. In these subjects, celecoxib did not alter the anticoagulant effect of warfarin as determined by prothrombin time. However, in post-marketing experience, serious bleeding events, some of which were fatal, have been reported, predominantly in the elderly, in association with increases in prothrombin time in patients receiving celecoxib concurrently with warfarin.
7.2 Lithium
In a study conducted in healthy subjects, mean steady-state lithium plasma levels increased approximately 17% in subjects receiving lithium 450 mg twice daily with celecoxib 200 mg twice daily as compared to subjects receiving lithium alone. Patients on lithium treatment should be closely monitored when celecoxib is introduced or withdrawn.
7.3 Aspirin
Celecoxib can be used with low-dose aspirin. However, concomitant administration of aspirin with celecoxib increases the rate of GI ulceration or other complications, compared to use of celecoxib alone [see Warnings and Precautions (5.1, 5.4) and Clinical Studies (14.6)].
Because of its lack of platelet effects, celecoxib is not a substitute for aspirin for cardiovascular prophylaxis [see Clinical Pharmacology (12.2)].
7.4 ACE-inhibitors and Angiotensin II Antagonists
Reports suggest that NSAIDs may diminish the antihypertensive effect of Angiotensin Converting Enzyme (ACE) inhibitors and angiotensin II antagonists. This interaction should be given consideration in patients taking celecoxibconcomitantly with ACE-inhibitors and angiotensin II antagonists [see Clinical Pharmacology (12.2)].
7.5 Fluconazole
Concomitant administration of fluconazole at 200 mg once daily resulted in a two-fold increase in celecoxib plasma concentration. This increase is due to the inhibition of celecoxib metabolism via P450 2C9 by fluconazole [see Clinical Pharmacology (12.3)]. Celecoxib should be introduced at the lowest recommended dose in patients receiving fluconazole.
7.6 Furosemide
Clinical studies, as well as post-marketing observations, have shown that NSAIDs can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Pregnancy category D from 30 weeks of gestation onward.
Teratogenic effects: Celecoxib at oral doses ≥150 mg/kg/day (approximately 2-fold human exposure at 200 mg twice daily as measured by AUC0 to 24), caused an increased incidence of ventricular septal defects, a rare event, and fetal alterations, such as ribs fused, sternebrae fused and sternebrae misshapen when rabbits were treated throughout organogenesis. A dose-dependent increase in diaphragmatic hernias was observed when rats were given celecoxib at oral doses ≥30 mg/kg/day (approximately 6-fold human exposure based on the AUC0 to 24 at 200 mg twice daily) throughout organogenesis. There are no studies in pregnant women. Celecoxib should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic effects: Celecoxib produced pre-implantation and post-implantation losses and reduced embryo/fetal survival in rats at oral dosages ≥50 mg/kg/day (approximately 6-fold human exposure based on the AUC0 to 24 at 200 mg twice daily). These changes are expected with inhibition of prostaglandin synthesis and are not the result of permanent alteration of female reproductive function, nor are they expected at clinical exposures. No studies have been conducted to evaluate the effect of celecoxib on the closure of the ductus arteriosus in humans. Therefore, use of celecoxib during the third trimester of pregnancy should be avoided.
8.2 Labor and Delivery
Celecoxib produced no evidence of delayed labor or parturition at oral doses up to 100 mg/kg in rats (approximately 7-fold human exposure as measured by the AUC0 to 24 at 200 mg BID). The effects of celecoxib on labor and delivery in pregnant women are unknown.
8.3 Nursing Mothers
Limited data from 3 published reports that included a total of 12 breastfeeding women showed low levels of celecoxib in breast milk. The calculated average daily infant dose was 10 to 40 mcg/kg/day, less than 1% of the weight-based therapeutic dose for a two-year old-child. A report of two breastfed infants 17 and 22 months of age did not show any adverse events. Caution should be exercised when celecoxibis administered to a nursing woman.
8.4 Pediatric Use
Celecoxibis approved for relief of the signs and symptoms of Juvenile Rheumatoid Arthritis in patients 2 years and older. Safety and efficacy have not been studied beyond six months in children. The long-term cardiovascular toxicity in children exposed to celecoxib has not been evaluated and it is unknown if long-term risks may be similar to that seen in adults exposed to celecoxib or other COX-2 selective and non-selective NSAIDs [(see Boxed Warning, Warnings and Precautions (5.12), and Clinical Studies (14.3)].
The use of celecoxib in patients 2 years to 17 years of age with pauciarticular, polyarticular course JRA or in patients with systemic onset JRA was studied in a 12-week, double-blind, active controlled, pharmacokinetic, safety and efficacy study, with a 12-week open-label extension. Celecoxib has not been studied in patients under the age of 2 years, in patients with body weight less than 10 kg (22 lbs), and in patients with active systemic features. Patients with systemic onset JRA (without active systemic features) appear to be at risk for the development of abnormal coagulation laboratory tests. In some patients with systemic onset JRA, both celecoxib and naproxen were associated with mild prolongation of activated partial thromboplastin time (APTT) but not prothrombin time (PT). NSAIDs including celecoxib should be used only with caution in patients with systemic onset JRA, due to the risk of disseminated intravascular coagulation. Patients with systemic onset JRA should be monitored for the development of abnormal coagulation tests [seeDosage and Administration (2.3), Warnings and Precautions (5.12), Adverse Reactions (6.3), Animal Toxicology (13.2), Clinical Studies (14.3)].
Alternative therapies for treatment of JRAshould be considered in pediatric patients identified to be CYP2C9 poor metabolizers [see Poor Metabolizers of CYP2C9 substrates (8.8)].
8.5 Geriatric Use
Of the total number of patients who received celecoxibin pre-approval clinical trials, more than 3,300 were 65 to74 years of age, while approximately 1,300 additional patients were 75 years and over. No substantial differences in effectiveness were observed between these subjects and younger subjects. In clinical studies comparing renal function as measured by the GFR, BUN and creatinine, and platelet function as measured by bleeding time and platelet aggregation, the results were not different between elderly and young volunteers. However, as with other NSAIDs, including those that selectively inhibit COX-2, there have been more spontaneous post-marketing reports of fatal GI events and acute renal failure in the elderly than in younger patients [see Warnings and Precautions (5.4, 5.6)].
8.6 Hepatic Insufficiency
The daily recommended dose of celecoxibcapsules in patients with moderate hepatic impairment (Child-Pugh Class B) should be reduced by 50%. The use of celecoxib in patients with severe hepatic impairment is not recommended [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
8.7 Renal Insufficiency
Celecoxibis not recommended in patients with severe renal in sufficiency [seeWarnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
8.8 Poor Metabolizers of CYP2C9 Substrates
Patients who are known or suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin, phenytoin) should be administered celecoxib with caution. Consider starting treatment at half the lowest recommended dose in poor metabolizers (i.e., CYP2C9*3/*3). Alternative management should be considered in JRA patients identified to be CYP2C9 poor metabolizers [see Dosage and Administration (2.6) and Clinical Pharmacology (12.5)].
-
10 OVERDOSAGE
No overdoses of celecoxib were reported during clinical trials. Doses up to 2,400 mg/day for up to 10 days in 12 patients did not result in serious toxicity. Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. No information is available regarding the removal of celecoxib by hemodialysis, but based on its high degree of plasma protein binding (>97%) dialysis is unlikely to be useful in overdose. Emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
-
11 DESCRIPTION
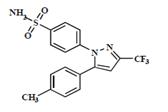
Celecoxib USP is chemically designated as 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole. The empirical formula is C17H14F3N3O2S, and the molecular weight is 381.38; the chemical structure is as follows:
Celecoxib oral capsules contain either50 mg, 100 mg, 200 mg or 400 mg of celecoxib, together with inactive ingredients including: croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, sodium lauryl sulfate, titanium dioxide and gelatin. Details of non-volatile components of the imprinting ink are given below.
50 mg capsule contains shellac, propylene glycol and red iron oxide.
100 mg capsule contains shellac, propylene glycol and FD & C Blue No. # 2 aluminum lake.
200 mg capsule contains shellac, propylene glycol and yellow iron oxide.
400 mg capsule contains shellac, propylene glycol, titanium dioxide, yellow iron oxide and FD & C Blue No. # 2 aluminum lake.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Celecoxib is a nonsteroidal anti-inflammatory drug that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of celecoxib is believed to be due to inhibition of prostaglandin synthesis, primarily via inhibition of cyclooxygenase-2 (COX-2), and at therapeutic concentrations in humans, celecoxib does not inhibit the cyclooxygenase-1 (COX-1) isoenzyme. In animal colon tumor models, celecoxib reduced the incidence and multiplicity of tumors.
12.2 Pharmacodynamics
Platelets: In clinical trials using normal volunteers, celecoxib at single doses up to 800 mg and multiple doses of 600 mg twice daily for up to 7 days duration (higher than recommended therapeutic doses) had no effect on reduction of platelet aggregation or increase in bleeding time. Because of its lack of platelet effects, celecoxib is not a substitute for aspirin for cardiovascular prophylaxis. It is not known if there are any effects of celecoxib on platelets that may contribute to the increased risk of serious cardiovascular thrombotic adverse events associated with the use of celecoxib.
Fluid Retention: Inhibition of PGE2 synthesis may lead to sodium and water retention through increased reabsorption in the renal medullary thick ascending loop of Henle and perhaps other segments of the distal nephron. In the collecting ducts, PGE2 appears to inhibit water reabsorption by counteracting the action of antidiuretic hormone.
12.3 Pharmacokinetics
Absorption:Peak plasma levels of celecoxib occur approximately 3 hrs after an oral dose. Under fasting conditions, both peak plasma levels (Cmax) and area under the curve (AUC) are roughly dose-proportional up to 200 mg BID; at higher doses there are less than proportional increases in Cmax and AUC [see Food Effects]. Absolute bioavailability studies have not been conducted. With multiple dosing, steady-state conditions are reached on or before Day 5. The pharmacokinetic parameters of celecoxib in a group of healthy subjects are shown in Table 3.
Table 3 Summary of Single Dose (200 mg) Disposition Kinetics of Celecoxib in Healthy Subjects1 Kinetics of Celecoxib in Healthy Subjects1
Mean (%CV) PK Parameter Values
Cmax, ng/mL
Tmax, hr
Effective t1/2, hr
Vss/F, L
CL/F, L/hr
705 (38)
2.8 (37)
11.2 (31)
429 (34)
27.7 (28)
1Subjects under fasting conditions (n=36, 19 to 52 yrs.)
Food Effects: When celecoxib capsules were taken with a high fat meal, peak plasma levels were delayed for about 1 to 2 hours with an increase in total absorption (AUC) of 10% to 20%. Under fasting conditions, at doses above 200 mg, there is less than a proportional increase in Cmax and AUC, which is thought to be due to the low solubility of the drug in aqueous media.
Coadministration of celecoxib with an aluminum- and magnesium-containing antacids resulted in a reduction in plasma celecoxib concentrations with a decrease of 37% in Cmax and 10% in AUC. Celecoxib, at doses up to 200 mg twice daily, can be administered without regard to timing of meals. Higher doses (400 mg twice daily) should be administered with food to improve absorption.
In healthy adult volunteers, the overall systemic exposure (AUC) of celecoxib was equivalent when celecoxib was administered as intact capsule or capsule contents sprinkled on applesauce. There were no significant alterations in Cmax, Tmaxor t1/2 after administration of capsule contents on applesauce [ see Dosage and Administration (2)].
Distribution: In healthy subjects, celecoxib is highly protein bound (~97%) within the clinical dose range. In vitro studies indicate that celecoxib binds primarily to albumin and, to a lesser extent, α1-acid glycoprotein. The apparent volume of distribution at steady state (Vss/F) is approximately 400 L, suggesting extensive distribution into the tissues. Celecoxib is not preferentially bound to red blood cells.
Metabolism: Celecoxib metabolism is primarily mediated via CYP2C9. Three metabolites, a primary alcohol, the corresponding carboxylic acid and its glucuronide conjugate, have been identified in human plasma. These metabolites are inactive as COX-1 or COX-2 inhibitors.
Excretion: Celecoxib is eliminated predominantly by hepatic metabolism with little (<3%) unchanged drug recovered in the urine and feces. Following a single oral dose of radiolabeled drug, approximately 57% of the dose was excreted in the feces and 27% was excreted into the urine. The primary metabolite in both urine and feces was the carboxylic acid metabolite (73% of dose) with low amounts of the glucuronide also appearing in the urine. It appears that the low solubility of the drug prolongs the absorption process making terminal half-life (t1/2) determinations more variable. The effective half-life is approximately 11 hours under fasted conditions. The apparent plasma clearance (CL/F) is about 500 mL/min.
Geriatric: At steady state, elderly subjects (over 65 years old) had a 40% higher Cmax and a 50% higher AUC compared to the young subjects. In elderly females, celecoxib Cmax and AUC are higher than those for elderly males, but these increases are predominantly due to lower body weight in elderly females. Dose adjustment in the elderly is not generally necessary. However, for patients of less than 50 kg in body weight, initiate therapy at the lowest recommended dose [see Dosage and Administration (2.6) and Use in Specific Populations (8.5)].
Pediatric: The steady state pharmacokinetics of celecoxib administered as an investigational oral suspension was evaluated in 152 JRA patients 2 years to 17 years of age weighing ≥10 kg with pauciarticular or polyarticular course JRA and in patients with systemic onset JRA. Population pharmacokinetic analysis indicated that the oral clearance (unadjusted for body weight) of celecoxib increases less than proportionally to increasing weight, with 10 kg and 25 kg patients predicted to have 40% and 24% lower clearance, respectively, compared with a 70 kg adult RA patient.
Twice-daily administration of 50 mg capsules to JRA patients weighing ≥12 to ≤25 kg and 100 mg capsules to JRA patients weighing >25 kg should achieve plasma concentrations similar to those observed in a clinical trial that demonstrated the non-inferiority of celecoxib to naproxen 7.5 mg/kg twice daily [see Dosage and Administration (2.3)]. Celecoxib has not been studied in JRA patients under the age of 2 years, in patients with body weight less than 10 kg (22 lbs), or beyond 24 weeks.
Race: Meta-analysis of pharmacokinetic studies has suggested an approximately 40% higher AUC of celecoxib in Blacks compared to Caucasians. The cause and clinical significance of this finding is unknown.
Hepatic Insufficiency: A pharmacokinetic study in subjects with mild (Child-Pugh Class A) and moderate (Child-Pugh Class B) hepatic impairment has shown that steady-state celecoxib AUC is increased about 40% and 180%, respectively, above that seen in healthy control subjects. Therefore, the daily recommended dose of Celecoxib should be reduced by approximately 50% in patients with moderate (Child-Pugh Class B) hepatic impairment. Patients with severe hepatic impairment (Child-Pugh Class C) have not been studied. The use of celecoxib in patients with severe hepatic impairment is not recommended [ see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Renal Insufficiency: In a cross-study comparison, celecoxib AUC was approximately 40% lower in patients with chronic renal insufficiency (GFR 35 to 60 mL/min) than that seen in subjects with normal renal function. No significant relationship was found between GFR and celecoxib clearance. Patients with severe renal insufficiency have not been studied. Similar to other NSAIDs, celecoxib is not recommended in patients with severe renal insufficiency [ see Warnings and Precautions (5.6)].
Drug interactions:
In vitro studies indicate that celecoxib is not an inhibitor of cytochrome P450 2C9, 2C19 or 3A4.
In vivo studies have shown the following:
Lithium: In a study conducted in healthy subjects, mean steady-state lithium plasma levels increased approximately 17% in subjects receiving lithium 450 mg twice daily with celecoxib 200 mg twice daily as compared to subjects receiving lithium alone [see Drug Interactions (7.2)].
Fluconazole: Concomitant administration of fluconazole at 200 mg once daily resulted in a two-fold increase in celecoxib plasma concentration. This increase is due to the inhibition of celecoxib metabolism via P450 2C9 by fluconazole [see Drug Interactions (7.5)].
Other Drugs: The effects of celecoxib on the pharmacokinetics and/or pharmacodynamics of glyburide, ketoconazole, methotrexate [see Drug Interactions (7.7)], phenytoin, and tolbutamide have been studied in vivo and clinically important interactions have not been found.
12.5 Pharmacogenomics
CYP2C9 activity is reduced in individuals with genetic polymorphisms that lead to reduced enzyme activity, such as those homozygous for the CYP2C9*2 and CYP2C9*3 polymorphisms. Limited data from 4 published reports that included a total of 8 subjects with the homozygous CYP2C9*3/*3 genotype showed celecoxib systemic levels that were 3- to 7-fold higher in these subjects compared to subjects with CYP2C9*1/*1 or *I/*3 genotypes. The pharmacokinetics of celecoxib have not been evaluated in subjects with other CYP2C9 polymorphisms, such as *2, *5, *6, *9 and *11. It is estimated that the frequency of the homozygous *3/*3 genotype is 0.3% to 1% in various ethnic groups [see Dosage and Administration (2.6), Use in Specific Populations (8.8)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Celecoxib was not carcinogenic in rats given oral doses up to 200 mg/kg for males and 10 mg/kg for females (approximately 2-to 4-fold the human exposure as measured by the AUC0 to 24 at 200 mg twice daily) or in mice given oral doses up to 25 mg/kg for males and 50 mg/kg for females (approximately equal to human exposure as measured by the AUC0 to 24 at 200 mg twice daily) for two years.
Celecoxib was not mutagenic in an Ames test and a mutation assay in Chinese hamster ovary (CHO) cells, nor clastogenic in a chromosome aberration assay in CHO cells and an in vivo micronucleus test in rat bone marrow.
Celecoxib did not impair male and female fertility in rats at oral doses up to 600 mg/kg/day (approximately 11-fold human exposure at 200 mg twice daily based on the AUC0 to 24).
13.2 Animal Toxicology
An increase in the incidence of background findings of spermatocele with or without secondary changes such as epididymal hypospermia as well as minimal to slight dilation of the seminiferous tubules was seen in the juvenile rat. These reproductive findings while apparently treatment-related did not increase in incidence or severity with dose and may indicate an exacerbation of a spontaneous condition. Similar reproductive findings were not observed in studies of juvenile or adult dogs or in adult rats treated with celecoxib. The clinical significance of this observation is unknown.
-
14 CLINICAL STUDIES
14.1 Osteoarthritis
Celecoxib has demonstrated significant reduction in joint pain compared to placebo. Celecoxib was evaluated for treatment of the signs and the symptoms of OA of the knee and hip in placebo- and active-controlled clinical trials of up to 12 weeks duration. In patients with OA, treatment with celecoxib 100 mg twice daily or 200 mg once daily resulted in improvement in WOMAC (Western Ontario and McMaster Universities) osteoarthritis index, a composite of pain, stiffness, and functional measures in OA. In three 12-week studies of pain accompanying OA flare, celecoxib doses of 100 mg twice daily and 200 mg twice daily provided significant reduction of pain within 24 to 48 hours of initiation of dosing. At doses of 100 mg twice daily or 200 mg twice daily the effectiveness of celecoxib was shown to be similar to that of naproxen 500 mg twice daily. Doses of 200 mg twice daily provided no additional benefit above that seen with 100 mg twice daily. A total daily dose of 200 mg has been shown to be equally effective whether administered as 100 mg twice daily or 200 mg once daily.
14.2 Rheumatoid Arthritis
Celecoxib has demonstrated significant reduction in joint tenderness/pain and joint swelling compared to placebo. Celecoxib was evaluated for treatment of the signs and symptoms of RA in placebo- and active-controlled clinical trials of up to 24 weeks in duration. Celecoxib was shown to be superior to placebo in these studies, using the ACR20 Responder Index, a composite of clinical, laboratory, and functional measures in RA.Celecoxib doses of 100 mg twice daily and 200 mg twice daily were similar in effectiveness and both were comparable to naproxen 500 mg twice daily.
Although celecoxib 100 mg twice daily and 200 mg twice daily provided similar overall effectiveness, some patients derived additional benefit from the 200 mg twice daily dose. Doses of 400 mg twice daily provided no additional benefit above that seen with 100 to 200 mg twice daily.
14.3 Juvenile Rheumatoid Arthritis
In a 12-week, randomized, double-blind active-controlled, parallel-group, multicenter, non-inferiority study, patients from 2 years to 17 years of age with pauciarticular, polyarticular course JRA or systemic onset JRA (with currently inactive systemic features), received one of the following treatments: celecoxib 3 mg/kg (to a maximum of 150 mg) twice daily; celecoxib 6 mg/kg (to a maximum of 300 mg) twice daily; or naproxen 7.5 mg/kg (to a maximum of 500 mg) twice daily. The response rates were based upon the JRA Definition of Improvement greater than or equal to 30% (JRA DOI 30) criterion, which is a composite of clinical, laboratory, and functional measures of JRA. The JRA DOI 30 response rates at week 12 were 69%, 80% and 67% in the celecoxib 3 mg/kg BID, celecoxib 6 mg/kg BID, and naproxen 7.5 mg/kg BID treatment groups, respectively.
The efficacy and safety of celecoxib for JRA have not been studied beyond six months. The long-term cardiovascular toxicity in children exposed to celecoxib has not been evaluated and it is unknown if the long-term risk may be similar to that seen in adults exposed to celecoxibor other COX-2 selective and non-selective NSAIDs [see Boxed Warning, Warnings and Precautions (5.12)].
14.4 Ankylosing Spondylitis
Celecoxib was evaluated in AS patients in two placebo- and active-controlled clinical trials of 6 and 12 weeks duration. Celecoxib at doses of 100 mg twice daily, 200 mg once daily and 400 mg once daily was shown to be statistically superior to placebo in these studies for all three co-primary efficacy measures assessing global pain intensity (Visual Analogue Scale), global disease activity (Visual Analogue Scale) and functional impairment (Bath Ankylosing Spondylitis Functional Index). In the 12-week study, there was no difference in the extent of improvement between the 200 mg and 400 mg celecoxib doses in a comparison of mean change from baseline, but there was a greater percentage of patients who responded to celecoxib 400 mg, 53%, than to celecoxib 200 mg, 44%, using the Assessment in Ankylosing Spondylitis response criteria (ASAS 20). The ASAS 20 defines a responder as improvement from baseline of at least 20% and an absolute improvement of at least 10 mm, on a 0 to 100 mm scale, in at least three of the four following domains: patient global pain, Bath Ankylosing Spondylitis Functional Index, and inflammation. The responder analysis also demonstrated no change in the responder rates beyond 6 weeks.
14.5 Analgesia, including Primary Dysmenorrhea
In acute analgesic models of post-oral surgery pain, post-orthopedic surgical pain, and primary dysmenorrhea, celecoxib relieved pain that was rated by patients as moderate to severe. Single doses [see Dosage and Administration (2.5)] of celecoxib provided pain relief within 60 minutes.
14.6 Special Studies
Adenomatous Polyp Prevention Studies:
Cardiovascular safety was evaluated in two randomized, double-blind, placebo-controlled, three year studies involving patients with Sporadic Adenomatous Polyps treated with celecoxib: the APC trial (Adenoma Prevention with Celecoxib) and the PreSAP trial (Prevention of Spontaneous Adenomatous Polyps). In the APC trial, there was a dose-related increase in the composite endpoint (adjudicated) of cardiovascular death, myocardial infarction, or stroke with celecoxib compared to placebo over 3 years of treatment. The PreSAP trial did not demonstrate a statistically significant increased risk for the same composite endpoint (adjudicated):
- In the APC trial, the hazard ratios compared to placebo for a composite endpoint (adjudicated) of cardiovascular death, myocardial infarction, or stroke were 3.4 (95% CI 1.4 to8.5) with celecoxib 400 mg twice daily and 2.8 (95% CI 1.1 to 7.2) with celecoxib 200 mg twice daily. Cumulative rates for this composite endpoint over 3 years were 3% (20/671 subjects) and 2.5% (17/685 subjects), respectively, compared to 0.9% (6/679 subjects) with placebo treatment. The increases in both celecoxib dose groups versus placebo-treated patients were mainly due to an increased incidence of myocardial infarction.
- In the PreSAP trial, the hazard ratio for this same composite endpoint (adjudicated) was 1.2 (95% CI 0.6 to2.4) with celecoxib 400 mg once daily compared to placebo. Cumulative rates for this composite endpoint over 3 years were 2.3% (21/933 subjects) and 1.9% (12/628 subjects), respectively.
Clinical trials of other COX-2 selective and non-selective NSAIDs of up to three-years duration have shown an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. As a result, all NSAIDs are considered potentially associated with this risk.
Celecoxib Long-Term Arthritis Safety Study (CLASS):
This was a prospective, long-term, safety outcome study conducted post-marketing in approximately 5,800 OA patients and 2,200 RA patients. Patients received celecoxib 400 mg twice daily (4-fold and 2-fold the recommended OA and RA doses, respectively), ibuprofen 800 mg three times daily or diclofenac 75 mg twice daily (common therapeutic doses). Median exposures for celecoxib(n = 3,987) and diclofenac (n = 1,996) were 9 months while ibuprofen (n = 1,985) was 6 months. The primary endpoint of this outcome study was the incidence of complicated ulcers (gastrointestinal bleeding, perforation or obstruction). Patients were allowed to take concomitant low-dose (≤ 325 mg/day) aspirin (ASA) for cardiovascular prophylaxis (ASA subgroups: celecoxib, n = 882; diclofenac, n = 445; ibuprofen, n = 412). Differences in the incidence of complicated ulcers between celecoxiband the combined group of ibuprofen and diclofenac were not statistically significant.
Patients on celecoxiband concomitant low-dose ASA (N=882) experienced 4-fold higher rates of complicated ulcers compared to those not on ASA (N=3,105). The Kaplan-Meier rate for complicated ulcers at 9 months was 1.12% versus 0.32% for those on low-dose ASA and those not on ASA, respectively [seeWarnings and Precautions (5.4)].
The estimated cumulative rates at 9 months of complicated and symptomatic ulcers for patients treated with celecoxib 400 mg twice daily are described in Table 4. Table 4 also displays results for patients less than or greater than 65 years of age. The difference in rates between celecoxib alone and celecoxib with ASA groups may be due to the higher risk for GI events in ASA users.
Table 4: Complicated and Symptomatic Ulcer Rates in Patients Taking celecoxib 400 mg Twice Daily (Kaplan-Meier Rates at 9 months [%]) Based on Risk Factors
All Patients
Celecoxib alone (n=3,105) 0.78
Celecoxib with ASA (n=882) 2.19
Patients <65 Years
Celecoxib alone (n=2,025) 0.47
Celecoxibwith ASA (n=403) 1.26
Patients ≥65 Years
Celecoxib alone (n=1,080) 1.4
Celecoxib with ASA (n=479) 3.06
In a small number of patients with a history of ulcer disease, the complicated and symptomatic ulcer rates in patients taking celecoxib alone or celecoxib with ASA were, respectively, 2.56% (n=243) and 6.85% (n=91) at 48 weeks. These results are to be expected in patients with a prior history of ulcer disease [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
Cardiovascular safety outcomes were also evaluated in the CLASS trial. Kaplan-Meier cumulative rates for investigator-reported serious cardiovascular thromboembolic adverse events (including MI, pulmonary embolism, deep venous thrombosis, unstable angina, transient ischemic attacks, and ischemic cerebrovascular accidents) demonstrated no differences between the celecoxib, diclofenac, or ibuprofen treatment groups. The cumulative rates in all patients at nine months for celecoxib, diclofenac, and ibuprofen were 1.2%, 1.4%, and 1.1%, respectively. The cumulative rates in non-ASA users at nine months in each of the three treatment groups were less than 1%. The cumulative rates for myocardial infarction in non-ASA users at nine months in each of the three treatment groups were less than 0.2%. There was no placebo group in the CLASS trial, which limits the ability to determine whether the three drugs tested had no increased risk of CV events or if they all increased the risk to a similar degree.
Endoscopic Studies: The correlation between findings of short-term endoscopic studies with celecoxiband the relative incidence of clinically significant serious upper GI events with long-term use has not been established. Serious clinically significant upper GI bleeding has been observed in patients receiving celecoxibin controlled and open-labeled trials [see Warnings and Precautions (5.4) and Clinical Studies (14.6)]
A randomized, double-blind study in 430 RA patients was conducted in which an endoscopic examination was performed at 6 months. The incidence of endoscopic ulcers in patients taking celecoxib200 mg twice daily was 4% vs. 15% for patients taking diclofenac SR 75 mg twice daily. However, celecoxib was not statistically different than diclofenac for clinically relevant GI outcomes in the CLASS trial [see Clinical Studies (14.6)].
The incidence of endoscopic ulcers was studied in two 12-week, placebo-controlled studies in 2,157 OA and RA patients in whom baseline endoscopies revealed no ulcers. There was no dose relationship for the incidence of gastroduodenal ulcers and the dose of celecoxib(50 mg to 400 mg twice daily). The incidence for naproxen 500 mg twice daily was 16.2% and 17.6% in the two studies, for placebo was 2% and 2.3%, and for all doses of celecoxib the incidence ranged between 2.7% to 5.9%. There have been no large, clinical outcome studies to compare clinically relevant GI outcomes with celecoxiband naproxen.
In the endoscopic studies, approximately 11% of patients were taking aspirin (≤ 325 mg/day). In the celecoxibgroups, the endoscopic ulcer rate appeared to be higher in aspirin users than in non-users. However, the increased rate of ulcers in these aspirin users was less than the endoscopic ulcer rates observed in the active comparator groups, with or without aspirin.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Celecoxib capsules 50 mg are opaque white/opaque white hard gelatin capsules size “5” having imprinting “134” on body with red ink and “A” on cap with red ink filled with white to off-white colored granular powder.
NDC: 46708-267-30 bottle of 30 capsules
NDC: 46708-267-60 bottle of 60 capsules
NDC: 46708-267-91 bottle of 1000 capsules
NDC: 46708-267-12 carton of 80 (10 x 8) unit-dose capsules
NDC: 46708-267-10 carton of 100 (10 x 10) unit-dose capsules
Celecoxib capsules 100 mg are opaque white/opaque white hard gelatin capsules size “3” having imprinting “135” on body with blue ink and “A” on cap with blue ink filled with white to off-white colored granular powder.
NDC: 46708-268-30 bottle of 30 capsules
NDC: 46708-268-60 bottle of 60 capsulesNDC: 46708-268-31 bottle of 100 capsules
NDC: 46708-268-71 bottle of 500 capsules
NDC: 46708-268-91 bottle of 1000 capsules
NDC: 46708-268-12 carton of 80 (10 x 8) unit-dose capsules
NDC: 46708-268-10 carton of 100 (10 x 10) unit-dose capsules
Celecoxib capsules 200 mg are opaque white/opaque white hard gelatin capsules size “1” having imprinting “136” on body with golden yellow ink and “A” on cap with golden yellow ink filled with white to off-white colored granular powder.
NDC: 46708-269-30 bottle of 30 capsules
NDC: 46708-269-60 bottle of 60 capsulesNDC: 46708-269-31 bottle of 100 capsules
NDC: 46708-269-71 bottle of 500 capsules
NDC: 46708-269-91 bottle of 1000 capsules
NDC: 46708-269-12 carton of 80 (10 x 8) unit-dose capsules
NDC: 46708-269-10 carton of 100 (10 x 10) unit-dose capsulesCelecoxib capsules 400 mg are opaque white/opaque white hard gelatin capsules size “00” having imprinting “137” on body with green ink and “A” on cap with green ink filled with white to off-white colored granular powder.
NDC: 46708-270-30 bottle of 30 capsules
NDC: 46708-270-60 bottle of 60 capsules
NDC: 46708-270-71 bottle of 500 capsules
NDC: 46708-270-10 carton of 100 (10 x 10) unit-dose capsules
NDC: 46708-270-14 carton of 126 (9 x 14) unit-dose capsules
Storage: Store at 20°-25°C (68°-77°F) excursions permitted to 15°-30°C (59°- 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Patients should be informed of the following information before initiating therapy with celecoxib and periodically during the course of ongoing therapy.
17.1 Medication Guide
Patients should be informed of the availability of a Medication Guide for NSAIDs that accompanies each prescription dispensed, and should be instructed to read the Medication Guide prior to using celecoxib.
17.2 Cardiovascular Effects
Patients should be informed that celecoxib may cause serious CV side effects such as MI or stroke, which may result in hospitalization and even death. Patients should be informed of the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and to seek immediate medical advice if they observe any of these signs or symptoms [seeWarnings and Precautions (5.1)].
Patients should be informed that celecoxib can lead to the onset of new hypertension or worsening of preexisting hypertension, and that celecoxibmay impair the response of some antihypertensive agents. Patients should be instructed on the proper follow up for monitoring of blood pressure [see Warnings and Precautions (5.2) and Drug Interactions (7.4)].
17.3 Gastrointestinal Effects
Patients should be informed that celecoxibcan cause gastrointestinal discomfort and more serious side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Patients should be informed of the signs and symptoms of ulcerations and bleeding, and to seek immediate medical advice if they observe any signs or symptoms that are indicative of these disorders, including epigastric pain, dyspepsia, melena, and hematemesis [see Warnings and Precautions (5.4)].
17.4 Hepatic Effects
Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). Patients should be instructed that they should stop therapy and seek immediate medical therapy if these signs and symptoms occur [see Warnings and Precautions (5.5), Use in Specific Populations (8.6)].
17.5 Adverse Skin Reactions
Patients should be informed that Celecoxib is a sulfonamide and can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be informed of the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and seek immediate medical advice when observing any indicative signs or symptoms.
Patients should be advised to stop celecoxibimmediately if they develop any type of rash and contact their physician as soon as possible.
Patients with prior history of sulfa allergy should not take celecoxib[see Warnings and Precautions (5.8)].
17.6 Weight Gain and Edema
Long-term administration of NSAIDs including celecoxibhas resulted in renal injury. Patients at greatest risk are those taking diuretics, ACE-inhibitors, angiotensin II antagonists, or with renal or liver dysfunction, heart failure, and the elderly [see Warnings and Precautions (5.3, 5.6), Use in Specific Populations (8)].
Patients should be instructed to promptly report to their physicians signs or symptoms of unexplained weight gain or edema following treatment with celecoxib[see Warnings and Precautions (5.3)].
17.7 Anaphylactoid Reactions
Patients should be informed of the signs and symptoms of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). Patients should be instructed to seek immediate emergency assistance if they develop any of these signs and symptoms [see Warnings and Precautions (5.7)].
17.8 Effects During Pregnancy
Patients should be informed that in late pregnancy celecoxibshould be avoided because it may cause premature closure of the ductus arteriosus [see Warnings and Precautions (5.9), Use in Specific Populations (8.1)].
17.9 Preexisting Asthma
Patients should be instructed to tell their physicians if they have a history of asthma or aspirin-sensitive asthma because the use of NSAIDs in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Patients with this form of aspirin sensitivity should be instructed not to take celecoxib. Patients with preexisting asthma should be instructed to seek immediate medical attention if their asthma worsens after taking celecoxib [see Warnings and Precautions (5.13)].
-
Medication Guide
Medication Guide
for
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
(See the end of this Medication Guide for a list of prescription NSAID medicines.)
What is the most important information I should know about medicines called
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?NSAID medicines may increase the chance of a heart attack or stroke that can lead to death.
This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID medicines should never be used right before or after a heart surgery called a “coronary artery bypass graft (CABG).”
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called “corticosteroids” and “anticoagulants”
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
- NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
different types of arthritis - menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all of your medical conditions.
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
- if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
Serious side effects include:
Other side effects include:
- heart attack
- stroke
- high blood pressure
- heart failure from body swelling (fluid retention)
- kidney problems including kidney failure
- bleeding and ulcers in the stomach and intestine
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
- liver problems including liver failure
- asthma attacks in people who have asthma
- stomach pain
- constipation
- diarrhea
- gas
- heartburn
- nausea
- vomiting
- dizziness
Get emergency help right away if you have any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
- nausea
- more tired or weaker than usual
- itching
- your skin or eyes look yellow
- stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- skin rash or blisters with fever
- unusual weight gain
- swelling of the arms and legs, hands and feet
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
Some of these NSAID medicines are sold in lower doses without a prescription (over –the–counter). Talk to your healthcare provider before using over–the–counter NSAIDs for more than 10 days.
NSAID medicines that need a prescription
Generic Name
Tradename
Celecoxib
Celebrex
Diclofenac
Cataflam, Voltaren, Arthrotec (combined with misoprostol)
Diflunisal
Dolobid
Etodolac
Lodine, Lodine XL
Fenoprofen
Nalfon, Nalfon 200
Flurbiprofen
Ansaid
Ibuprofen
Motrin, Tab-Profen, Vicoprofen* (combined with hydrocodone), Combunox (combined with oxycodone)
Indomethacin
Indocin, Indocin SR, Indo-Lemmon, Indomethagan
Ketoprofen
Oruvail
Ketorolac
Toradol
Mefenamic Acid
Ponstel
Meloxicam
Mobic
Nabumetone
Relafen
Naproxen
Naprosyn, Anaprox, Anaprox DS, EC-Naproxyn, Naprelan, Naprapac (copackaged with lansoprazole)
Oxaprozin
Daypro
Piroxicam
Feldene
Sulindac
Clinoril
Tolmetin
Tolectin,TolectinDS, Tolectin 600
*Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAIDs, and is usually used for less than 10 days to treat pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, IndiaRevised: 09/2019
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-50 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-100 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-200 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-400 mg
-
INGREDIENTS AND APPEARANCE
CELECOXIB
celecoxib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-267 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CELECOXIB (UNII: JCX84Q7J1L) (CELECOXIB - UNII:JCX84Q7J1L) CELECOXIB 50 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 11mm Flavor Imprint Code A;134 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-267-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 2 NDC: 46708-267-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 3 NDC: 46708-267-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 4 NDC: 46708-267-12 80 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 5 NDC: 46708-267-10 100 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204519 08/22/2015 CELECOXIB
celecoxib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-268 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CELECOXIB (UNII: JCX84Q7J1L) (CELECOXIB - UNII:JCX84Q7J1L) CELECOXIB 100 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code A;135 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-268-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 2 NDC: 46708-268-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 3 NDC: 46708-268-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 4 NDC: 46708-268-12 80 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 5 NDC: 46708-268-10 100 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 6 NDC: 46708-268-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 7 NDC: 46708-268-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204519 08/22/2015 CELECOXIB
celecoxib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-269 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CELECOXIB (UNII: JCX84Q7J1L) (CELECOXIB - UNII:JCX84Q7J1L) CELECOXIB 200 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code A;136 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-269-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 2 NDC: 46708-269-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 3 NDC: 46708-269-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 4 NDC: 46708-269-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 5 NDC: 46708-269-12 80 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 6 NDC: 46708-269-10 100 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 7 NDC: 46708-269-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204519 08/22/2015 CELECOXIB
celecoxib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-270 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CELECOXIB (UNII: JCX84Q7J1L) (CELECOXIB - UNII:JCX84Q7J1L) CELECOXIB 400 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code A;137 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-270-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 2 NDC: 46708-270-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 3 NDC: 46708-270-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/22/2015 4 NDC: 46708-270-10 100 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 5 NDC: 46708-270-14 126 in 1 CARTON; Type 0: Not a Combination Product 08/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204519 08/22/2015 Labeler - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(46708-267, 46708-268, 46708-269, 46708-270)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.