Utopic by Artesa Labs, LLC UTOPIC- urea cream
Utopic by
Drug Labeling and Warnings
Utopic by is a Prescription medication manufactured, distributed, or labeled by Artesa Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
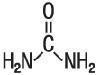
- DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- INDICATIONS:
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General: This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients: Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes.
Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed.
Pregnancy:Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
- ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
-
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
NOTICE: Protect from freezing and excessive heat. The product may tend to darken in color on storage. The discoloration does not impair the efficacy or safety of the product. Keep bottle tightly closed.
- HOW SUPPLIED:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UTOPIC
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57893-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 410 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57893-301-08 227 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/15/2011 2 NDC: 57893-301-09 9 in 1 CARTON 08/15/2011 01/31/2020 2 10 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 57893-301-01 10 in 1 CARTON 02/01/2018 3 NDC: 57893-301-04 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/15/2011 Labeler - Artesa Labs, LLC (078786339)
Trademark Results [Utopic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 UTOPIC 90505333 not registered Live/Pending |
Utopic, LLC 2021-02-02 |
 UTOPIC 85941941 4499574 Live/Registered |
BKK Pharmaceuticals, LLC 2013-05-24 |
 UTOPIC 85342626 not registered Dead/Abandoned |
Derrick R. Nelson 2011-06-09 |
 UTOPIC 85338949 not registered Dead/Abandoned |
Derrick R. Nelson 2011-06-06 |
 UTOPIC 77853104 4077504 Dead/Cancelled |
Utopic, LLC 2009-10-20 |
 UTOPIC 77853078 4077503 Dead/Cancelled |
Utopic, LLC 2009-10-20 |
 UTOPIC 77853063 4077502 Dead/Cancelled |
Utopic, LLC 2009-10-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.