FULVESTRANT injection, solution
FULVESTRANT by
Drug Labeling and Warnings
FULVESTRANT by is a Prescription medication manufactured, distributed, or labeled by Zydus Lifesciences Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
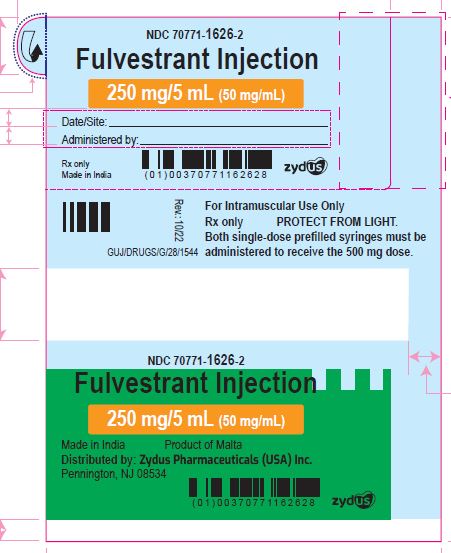
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 70771-1626-2
Fulvestrant Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular Use Only
Both single-dose prefilled syringes must be administered to receive the 500 mg dose.
PROTECT FROM LIGHT
Rx only
Made in India
NDC: 70771-1626-8
Fulvestrant Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular Use Only
This carton contains a total of 500 mg fulvestrant in TWO single-dose prefilled syringes each containing 250 mg/5 mL, and two Safety Glide™ shielding intramuscular injection needles.
Discard each syringe after use.
Both single-dose prefilled syringes must be administered to receive the 500 mg dose.
STORAGE: REFRIGERATE 2° to 8°C (36° to 46°F). TO PROTECT FROM LIGHT, STORE IN THE ORIGINAL CARTON UNTIL TIME OF USE.
Contains 2 Single-Dose Prefilled Syringes
Rx only

-
INGREDIENTS AND APPEARANCE
FULVESTRANT
fulvestrant injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70771-1626 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FULVESTRANT (UNII: 22X328QOC4) (FULVESTRANT - UNII:22X328QOC4) FULVESTRANT 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZYL BENZOATE (UNII: N863NB338G) CASTOR OIL (UNII: D5340Y2I9G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70771-1626-8 2 in 1 CARTON 12/14/2021 1 NDC: 70771-1626-2 5 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215234 12/14/2021 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 677604722 ANALYSIS(70771-1626) , MANUFACTURE(70771-1626)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.