SKEDERM VITAMIN C X30 POWER SERUM by Skederm Inc. / Kolmar Korea Co., Ltd. 70209-003_Deactivation
SKEDERM VITAMIN C X30 POWER SERUM by
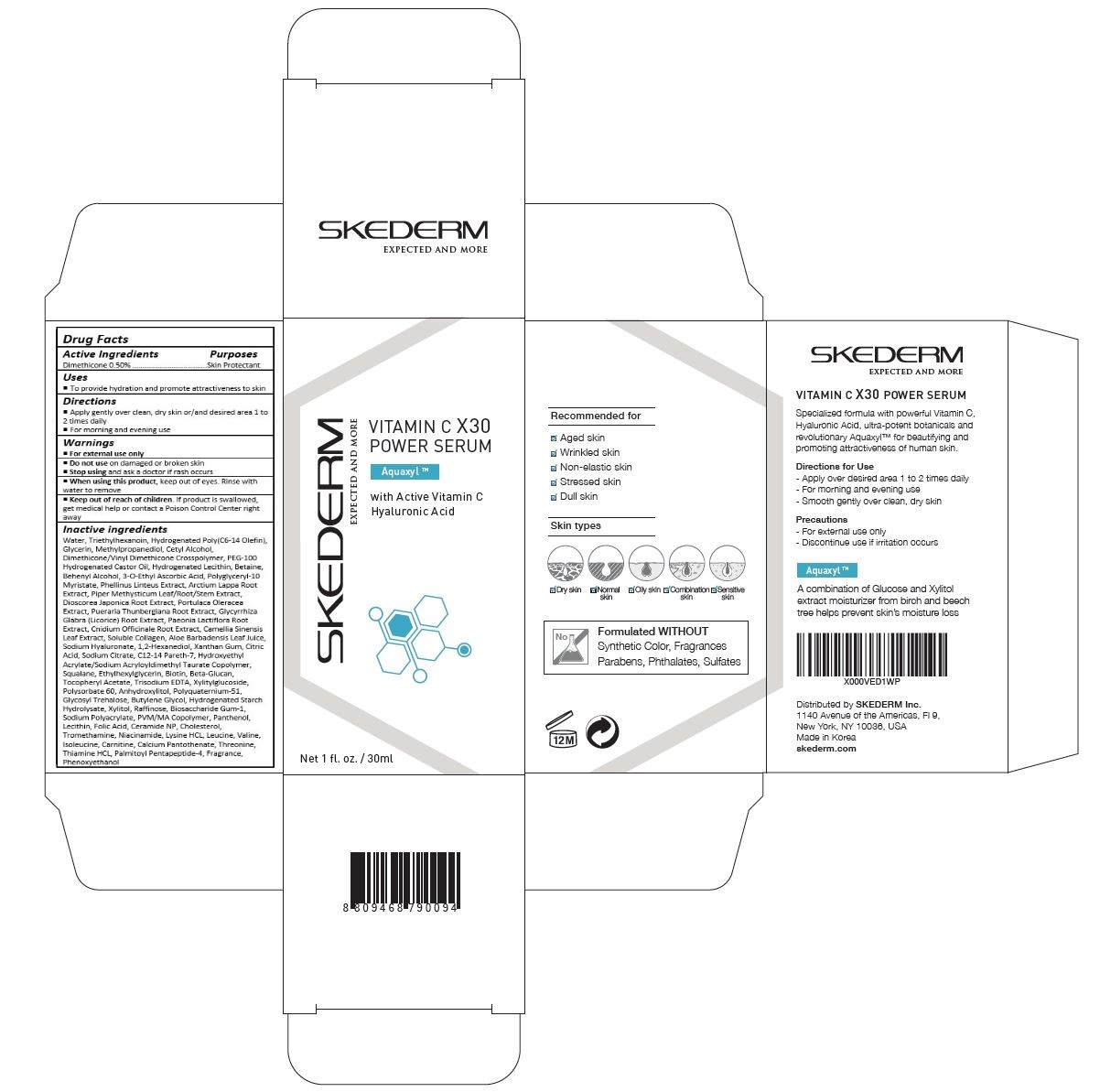
Drug Labeling and Warnings
SKEDERM VITAMIN C X30 POWER SERUM by is a Otc medication manufactured, distributed, or labeled by Skederm Inc., Kolmar Korea Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SKEDERM VITAMIN C X30 POWER SERUM- dimethicone cream
Skederm Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
70209-003_Deactivation
Apply gently over clean, dry skin or/and desired area 1 to 2 times daily
For morning and evening use
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop using and ask a doctor if rash occurs.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
Water, Triethylhexanoin, Hydrogenated Poly(C6-14 Olefin), Cetyl Alcohol, Methylpropanediol, Dimethicone/Vinyl Dimethicone Crosspolymer, PEG-100 Hydrogenated Castor Oil, Hydrogenated Lecithin, 3-O-Ethyl Ascorbic Acid, Behenyl Alcohol, Betaine, Polyglyceryl-10 Myristate, Piper Methysticum Leaf/Root/Stem Extract, Aloe Barbadensis Leaf Juice, 1,2-Hexanediol, Arctium Lappa Root Extract, Phellinus Linteus Extract, Soluble Collagen, Phenoxyethanol, Xanthan Gum, Dioscorea Japonica Root Extract, Citric Acid, Sodium Citrate, C12-14 Pareth-7, Portulaca Oleracea Extract, Pueraria Thunbergiana Root Extract, Cnidium Officinale Root Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Paeonia Lactiflora Root Extract, Squalane, Ethylhexylglycerin, Fragrance, Tocopheryl Acetate, Trisodium EDTA, Polysorbate 60, Polyquaternium-51, Glycosyl trehalose, Sodium Hyaluronate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydrogenated Starch Hydrolysate, Biosaccharide Gum-1, PVM/MA Copolymer, Raffinose, Sodium Polyacrylate, Lecithin
| SKEDERM VITAMIN C X30 POWER SERUM
dimethicone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Skederm Inc. (079981781) |
| Registrant - Skederm Inc. (079981781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kolmar Korea Co., Ltd. | 689512611 | manufacture(70209-003) | |