These highlights do not include all the information needed to use RISVAN ® safely and effectively. See full prescribing information for RISVAN. RISVAN ® (risperidone) for extended-release injectable suspension, for intramuscular use Initial U.S. Approval: 1993
RISVAN by
Drug Labeling and Warnings
RISVAN by is a Prescription medication manufactured, distributed, or labeled by Laboratorios Farmaceuticos Rovi, S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RISVAN- risperidone injection, powder, for suspension, extended release
Laboratorios Farmaceuticos Rovi, S.A.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RISVAN
® safely and effectively. See full prescribing information for RISVAN.
RISVAN ® (risperidone) for extended-release injectable suspension, for intramuscular use Initial U.S. Approval: 1993 WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSISSee full prescribing information for complete boxed warning.Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. RISVAN is not approved for use in patients with dementia-related psychosis. ( 5.1) INDICATIONS AND USAGERISVAN is an atypical antipsychotic indicated for the treatment of schizophrenia in adults. ( 1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSFor extended-release injectable suspension: 75 mg and 100 mg risperidone. ( 3) CONTRAINDICATIONSKnown hypersensitivity to risperidone, paliperidone, or other components of RISVAN. ( 4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most frequently reported adverse reactions in clinical trials (≥ 5% and twice placebo): hyperprolactinaemia, blood prolactin increased, akathisia, headache, sedation (including somnolence), weight increased, injection site pain, and alanine aminotransferase increased. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Laboratorios Farmacéuticos Rovi at 1-888-703-0896 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSPregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. ( 8.1) Revised: 3/2024 |
FULL PRESCRIBING INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. RISVAN is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions ( 5.1)] .
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
RISVAN must be administered by a healthcare professional as a deltoid or gluteal intramuscular injection. Do not administer RISVAN by any other route.
For detailed preparation and administration instructions, see Dosage and Administration (2.5).
2.2 Recommended Dosage
Initiate RISVAN at a dose of 75 mg or 100 mg once monthly by deltoid or gluteal intramuscular injection. Do not administer more than one dose (75 mg or 100 mg total) per month.
For patients who have never taken risperidone, establish tolerability with oral risperidone prior to initiating RISVAN.
For patients switching from oral risperidone:
- 3 mg of oral risperidone per day, administer a 75 mg RISVAN dose one day after the last oral risperidone dose.
- 4 mg of oral risperidone per day, administer a 100 mg RISVAN dose one day after the last oral risperidone dose.
Patients who are on stable oral risperidone doses lower than 3 mg per day or higher than 4 mg per day may not be candidates for RISVAN [see Clinical Pharmacology ( 12.3) and Clinical Studies ( 14)].
Neither a loading dose nor supplemental oral risperidone is recommended. Administer subsequent RISVAN doses once monthly as a deltoid or gluteal intramuscular injection.
When a dose of RISVAN is missed, administer the next RISVAN dose as soon as possible. Do not administer more than one RISVAN dose per month.
2.3 Dosage Recommendations for Patients with Renal or Hepatic Impairment
Prior to initiating treatment with RISVAN in patients with renal or hepatic impairment, titrate with oral risperidone to atleast 3 mg daily. Following oral titration, and based on clinical response and tolerability, the recommended dosage of RISVAN is 75 mg once monthly [see Use in Specific Populations ( 8.6, 8.7) and Clinical Pharmacology ( 12.3)].
2.4 Dosage Modifications for Concomitant Use with Strong CYP2D6 Inhibitors and Strong CYP3A4 Inducers
Co-administration with Strong CYP2D6 Inhibitors
Between 2 to 4 weeks prior to initiating a strong CYP2D6 inhibitor, switch patients (if applicable) to the lowest RISVAN dosage (75 mg once monthly) to adjust for the expected increase in plasma concentrations of risperidone [see Drug Interactions ( 7.1)] .
When a strong CYP2D6 inhibitor is initiated in patients receiving RISVAN 75 mg, continue treatment with 75 mg unless clinical judgment necessitates interruption of RISVAN treatment [see Drug Interactions ( 7.1)] .
Co-administration with Strong CYP3A4 Inducers
With concomitant use of RISVAN 75 mg and strong CYP3A4 inducers, increase the RISVAN dosage to 100 mg once monthly. In patients receiving RISVAN 100 mg, additional oral risperidone therapy may need to be considered.
Upon discontinuation of a strong CYP3A4 inducer in a patients receiving RISVAN 100 mg once monthly, re-evaluate the dosage of RISVAN or any additional oral risperidone therapy and, if necessary, decrease to adjust for the expected increase in plasma concentration of risperidone.
Upon discontinuation of a strong CYP3A4 inducer in a patient receiving RISVAN 75 mg once monthly, continue treatment with the 75 mg dose unless clinical judgment necessitates interruption of RISVAN treatment [see Drug Interactions ( 7.1)] .
2.5 Preparation and Administration Instructions
- To be prepared and administered by a healthcare professional only.
- Read the instructions for preparation and administration below and consider referring to the separate Healthcare Provider “Instructions for Use” for preparation and administration considerations.
- Label the administration syringe immediately after reconstitution, then administer immediately.
- For deltoid or gluteal intramuscular injection only. Do not inject by any other route. As a universal precaution, always wear gloves.
- Do not substitute any component of the drug kit.
STEP 1 CHECK CONTENTS
Working on a clean surface, open the pouches and discard the desiccant .
Each carton of RISVAN contains:
- One pouch with a RISVAN prefilled syringe with a WHITE plunger rod and WHITE finger flange.
- One pouch with SOLVENT for RISVAN prefilled syringe with a TRANSPARENT plunger rod and a colored finger flange.
- Two administration needles 21G, 1 inch for deltoid (green cap) and a 20G, 2 inch for gluteus (yellow cap).
Visually inspect RISVAN for particles and discoloration prior to administration. Discard the kit if any component is damaged.
INSPECT SOLVENT SYRINGE
ENSURE that SOLVENT syringe content flows normally as a liquid. The solvent freezes at 66 ºF. If the solvent is frozen or partially frozen, warm it at room temperature until it flows normally.
DISLODGE POWDER SYRINGE
TAP the RISVAN syringe to dislodge potential packed powder near the cap.
STEP 2 CONNECT THE SYRINGES
UNCAP SYRINGES IN UPRIGHT POSITION
Hold both syringes in upright position to prevent loss of product.
PULL the cap off the Solvent syringe.
TWIST and PULL the Powder syringe cap off.
- Make sure that Powder syringeis in the upright position to prevent loss of product.
CONNECT THE SYRINGES
Pick the solvent syringe that has the S, or slightly tilt it vertically.
TWIST the syringes together until you feel a slight resistance.
Make sure that Powder syringe R is in the upright position to prevent loss of product.
STEP 3 MIX THE CONTENTS
- STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
- PUSH VIGOROUSLY the Solvent content S towards the Powder syringe.
- DO NOT WAIT for powder wetting and QUICKLY start mixing contents by pushing the plungers FAST and alternately for 100 pushes (2 pushes within 1 second, approximately 1 minute).
- ENSUREmedication is passing between both syringes for proper mixing: medication is viscous and you will need to apply force when pressing on the plunger rods.
- Mix for at least 100 pushes by doing alternately 1 followed by 2
- Make sure medication is passing between both syringes
When medication is correctly mixed, the appearance will be a uniform suspension off white to yellowish color andthick consistency.
- Proceed immediately to prepare the injection for administration.
STEP 4 PREPARE INJECTION SYRINGE
TRANSFER MEDICATION
Place downward pressure on the R syringe that has the colored flange.
- Make sure all the content is transferred.
DETACH SYRINGES
Once the medication is fully transferred, separate the two syringes by untwisting.
Proceed immediately to prepare the injection syringe for administration.
The injection must be given within 15 minutes after reconstitution.
ATTACH THE SAFETY NEEDLE
Choose the proper needle:
- Deltoid: 21G, 1 inch for deltoid (green cap).
- Gluteus: 20G, 2 inch for gluteus (yellow cap).
Attach it using a clockwise twisting motion. Do not over-tighten.
REMOVE EXCESS AIR
Remove needle cover and push out the excess air (only big bubbles) from the syringe barrel.
DO NOT expel any drops of medication
If you see the medication appearing at the needle tip, pull slightly back on the plunger to prevent medication spillage.
STEP 5 ADMINISTER AND DISPOSE
INJECT MEDICATION
Insert the needle fully into the deltoid or gluteal muscle. DO NOT INJECT BY ANY OTHER ROUTE.
- WARNINGS
- THICK MEDICATION, INJECT IT SLOWLY AND STEADILY. MAKE SURE TO FULLY INJECT IT.
- The injection time is longer than usual due to the viscosity of the medication.
- Wait a few seconds before removing the needle.
DISPOSE OF NEEDLE AND SYRINGE.
Cover the needle by pressing on the needle guard using a finger or a flat surface and dispose immediately.
3 DOSAGE FORMS AND STRENGTHS
RISVAN (risperidone) for extended-release suspension is available in strengths of 75 mg and 100 mg. Each strength is provided as a kit which includes: one pre-filled syringe containing a white to white-yellowish powder in a sealed pouch with a desiccant, one pre-filled syringe containing transparent and colorless solvent in a sealed pouch with a desiccant, one 20-gauge, 2-inch needle for gluteal administration and one 21-gauge, 1 inch needle for deltoid administration.
4 CONTRAINDICATIONS
RISVAN is contraindicated in patients with a known hypersensitivity to either risperidone, its metabolite, paliperidone or to any of the components in the formulation. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone [see Adverse Reactions ( 6.2)] .
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
In two of four placebo-controlled trials in elderly patients with dementia-related psychosis, a higher incidence of mortality was observed in patients treated with furosemide plus oral risperidone when compared to patients treated with oral risperidone alone or with placebo plus furosemide. No pathological mechanism has been identified to explain this finding, and no consistent pattern for cause of death was observed.
RISVAN is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions ( 5.2)] .
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
Cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities, were reported in patients (mean age 85 years; range 73 to 97) in trials of oral risperidone in elderly patients with dementia-related psychosis. In placebo-controlled trials, there was a significantly higher incidence of cerebrovascular adverse reactions in patients treated with oral risperidone compared to patients given placebo. RISVAN is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome (NMS)
NMS, a potentially fatal symptom complex, has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status including delirium, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
If NMS is suspected, immediately discontinue RISVAN and provide symptomatic treatment and monitoring.
5.4 Tardive Dyskinesia
Tardive dyskinesia, a syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible is believed to increase as the duration of treatment and the total cumulative dose. The syndrome can develop, after relatively brief treatment periods, even at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, RISVAN should be prescribed in a manner most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: 1) who suffer from a chronic illness that is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response should. Periodically reassess the need for continued treatment.
If signs and symptoms of tardive dyskinesia appear in a patient treated with RISVAN, drug discontinuation should be considered. However, some patients may require treatment with RISVAN despite the presence of the syndrome.
5.5 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics, including risperidone. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related events in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics, including RISVAN, should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics, including RISVAN, should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics, including RISVAN, should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics, including RISVAN, should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic, including risperidone, was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of risperidone.
Data from a 12-week double-blind, placebo-controlled study with RISVAN in adults with schizophrenia are presented in Table 1.
| RISVAN
75 mg N = 144 | RISVAN
100 mg N = 146 | Placebo
N = 147 |
|
| Serum Glucose, mg/dL, mean†
Mean Change from Baseline to End of Treatment | 6.0 (n= 129) | 1.80 (n = 125) | 0.3 (n = 119) |
| Glucose, > 126 mg/dL
Proportion of patients with Postbaseline Abnormal Values ‡ |
22/142 (15.5%) | 27/142 (19%) | 8/109 (7.3%) |
†The “n”s in the Serum Glucose mean row are the number of patients with data at baseline and end of treatment visits.
‡ Data shown as number of patients with at least one postbaseline value as denominator and number of patients satisfying the predefined criterion as numerator
In longer-term, controlled and uncontrolled studies, oral risperidone was associated with a mean change in glucose of +2.8 mg/dL at Week 24 (n=151) and +4.1 mg/dL at Week 48 (n=50).
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics, including risperidone. Before or soon after initiation of antipsychotic medications, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
Weight Gain
Weight gain has been observed with atypical antipsychotic use. Monitor weight at baseline and frequently thereafter.
Data from a 12-week double-blind, placebo-controlled study with RISVAN in adults with schizophrenia are presented in Table 2.
| RISVAN 75mg | RISVAN 100mg | Placebo | |
|
Weight (kg) † Change from baseline |
n=129 2.2 |
n=126 2.0 |
n=121 0.2 |
|
Weight (kg) ‡ ≥ 7% increase from baseline | 15/129 (11.6%) | 20/126 (15.9%) | 5/121 (4.1%) |
†The “n”s in the Weight Change mean row are the number of patients with data at baseline and end of treatment visits.
‡ Data shown as number of patients with at least one postbaseline value as denominator and number of patients satisfying the predefined criterion as numerator.
Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended. In an open-label, 12-month long-term safety study, mean weight increased by approximately 0.4 kg from baseline to Day 85 and 1.1 kg from baseline to Day 365 in patients receiving RISVAN.
5.6 Hyperprolactinemia
As with other drugs that antagonize dopamine D2 receptors, risperidone elevates prolactin levels and the elevation persists during chronic administration. Risperidone is associated with higher levels of prolactin elevation than other antipsychotic agents.
Hyperprolactinemia may suppress hypothalamic gonadotropin releasing hormone, resulting in reduced pituitary gonadotropin secretion. This may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with previously detected breast cancer. An increase in pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats [see Nonclinical Toxicology (13.1)]. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans; the available evidence is considered too limited to be conclusive at this time.
5.7 Orthostatic Hypotension and Syncope
RISVAN may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope, probably reflecting its alpha-adrenergic antagonistic properties. Syncope was reported in 0.2% (6/2607) of patients treated with oral risperidone in Phase 2 and 3 studies in adults with schizophrenia.
RISVAN should be used with particular caution in ( 1) patients with known cardiovascular disease (history of myocardial infarction or ischemia, heart failure, or conduction abnormalities), cerebrovascular disease, and conditions which would predispose patients to hypotension, e.g., dehydration and hypovolemia, and ( 2) in the elderly and patients with renal or hepatic impairment. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs. Clinically significant hypotension has been observed with concomitant use of oral risperidone and antihypertensive medication.
5.8 Falls
Antipsychotics, including RISVAN, may cause somnolence, postural hypotension, motor and sensory instability which may lead to falls and, consequently, fractures or other fall-related injuries. Somnolence, postural hypotension, motor and sensory instability have been reported with the use of risperidone. For patients, particularly the elderly, with diseases, conditions, or medications that could exacerbate these effects, assess the risk of falls when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.9 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trial and/or postmarketing experience, events of leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including risperidone. Agranulocytosis has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug-induced leukopenia/neutropenia. In patients with a history of a clinically significant low WBC/ANC or a drug-induced leukopenia/neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of RISVAN at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Discontinue RISVAN in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery.
5.10 Potential for Cognitive and Motor Impairment
Somnolence, sedation, and dizziness were reported as adverse reactions in patients treated with RISVAN [see Adverse Reactions (6.1)]. Antipsychotics, including RISVAN, have the potential to impair judgment, thinking, or motor skills.
In a 12-week, double-blind, placebo-controlled study, sedation (including somnolence) was reported by 4%, 6%, and 3% of patients treated with 75 mg of RISVAN, 100 mg of RISVAN, and placebo, respectively. Dizziness was reported by 4%, 4%, and 3% of 75 mg of RISVAN, 100 mg of RISVAN, and placebo, respectively.
Patients should be cautioned about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that RISVAN does not adversely affect them.
5.11 Seizures
During premarketing studies of oral risperidone in adult patients with schizophrenia, seizures occurred in 0.3% of patients (9 out of 2,607 patients), two in association with hyponatremia. Use RISVAN cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold.
5.12 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer's dementia. Antipsychotic drugs, including RISVAN, should be used cautiously in patients at risk for aspiration .
5.13 Priapism
Priapism has been reported during postmarketing surveillance for other risperidone products. Severe priapism may require surgical intervention.
5.14 Body Temperature Regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with oral risperidone use. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use RISVAN with caution in patients who may experience these conditions.
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions ( 5.1)]
- Cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis [see Warnings and Precautions ( 5.2)]
- Neuroleptic malignant syndrome (NMS) [see Warnings and Precautions ( 5.3)]
- Tardive dyskinesia [see Warnings and Precautions ( 5.4)]
- Metabolic changes [see Warnings and Precautions ( 5.5)]
- Hyperprolactinemia [see Warnings and Precautions ( 5.6)]
- Orthostatic hypotension and syncope [see Warnings and Precautions ( 5.7)]
- Falls [see Warnings and Precautions ( 5.8)]
- Leukopenia, neutropenia, and agranulocytosis [see Warnings and Precautions ( 5.9)]
- Potential for cognitive and motor impairment [see Warnings and Precautions ( 5.10)]
- Seizures [see Warnings and Precautions ( 5.11)]
- Dysphagia [see Warnings and Precautions ( 5.12)]
- Priapism [see Warnings and Precautions ( 5.13)]
- Body temperature regulation [see Warnings and Precautions ( 5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RISVAN for the treatment of schizophrenia in adults is based on adequate and well controlled studies of oral risperidone in studies of patients with schizophrenia and other indications as well as in one 12-week placebo-controlled trial of RISVAN in adult patients with schizophrenia [see Clinical Studies ( 14)] .
The safety of RISVAN was evaluated in a total of 562 adult patients with schizophrenia who received at least 1 dose of RISVAN during the clinical development program. Of the 386 patients with schizophrenia who received one dose of Risvan during the 12-week, placebo-controlled trial, 168 patients received at least 13 doses of RISVAN during the double-blind and open label extension (OLE) phases. During double-blind period, RISVAN 75 mg was administered to 144 patients and RISVAN 100 mg to 146 patients. During the OLE period, RISVAN 75 mg was administered to 116 patients and RISVAN 100 mg to 99 patients.
Adverse Reactions in the 12-Week Placebo-Controlled Trial in Adults with Schizophrenia
The safety data presented below are derived from the 12-week double-blind placebo-controlled study of RISVAN in adult patients with schizophrenia.
Adverse reactions that led to discontinuation in RISVAN-treated patients in the 12-week placebo-controlled trial in adults with schizophrenia include abscess limb (0.3%), skin infection (0.3%), fall (0.3%), humerus fracture (0.3%), liver function test increased (0.3%), neutrophil count decreased (0.3%), mental impairment (0.3%), erectile dysfunction (0.3%), galactorrhea (0.3%), lactation disorder (0.3%), and pruritis (0.3%).
The most frequently reported adverse reactions (≥5% and twice placebo) are blood prolactin increase, hyperprolactinemia, akathisia, headache, sedation (including somnolence), weight increased, injection site pain, and alanine aminotransferase increased.
Table 3 shows the incidence of adverse reactions that occurred in ≥2% of patients treated with RISVAN and at a greater frequency than placebo.
|
System Organ Class Preferred Term |
RISVAN 75 mg (n = 146) % |
RISVAN 100 mg (n = 144) % |
Placebo (n = 147) % |
| Cardiac disorders | |||
| Tachycardia | 1 | 3 | 0 |
| Endocrine disorders | |||
| Hyperprolactinemia | 6 | 9 | 1 |
| Gastrointestinal disorders | |||
| Constipation | 3 | 1 | 1 |
|
General disorders and administration site conditions | |||
| Injection site pain | 6 | 3 | 3 |
| Infections and infestations | |||
| Nasopharyngitis | 4 | 3 | 0 |
| Investigations | |||
| Blood prolactin increased | 9 | 14 | 0 |
| Weight increased | 7 | 6 | 2 |
| Alanine aminotransferase increased | 3 | 5 | 2 |
| Aspartate aminotransferase increased | 1 | 3 | 2 |
| Blood triglycerides increased | 3 | 2 | 1 |
| Blood creatine phosphokinase increased | 2 | 1 | 1 |
| Blood cholesterol increased | 0 | 2 | 0 |
| Musculoskeletal and connective tissue disorders | |||
| Myalgia | 2 | 1 | 0 |
| Muscle tightness | 2 | 0 | 0 |
| Nervous system disorders | |||
| Headache | 10 | 8 | 3 |
| Akathisia | 4 | 8 | 2 |
| Sedation 1 | 4 | 6 | 3 |
| Dizziness | 4 | 4 | 3 |
| Dystonia 2 | 4 | 4 | 1 |
1 Sedation includes sedation and somnolence
2 Dystonia includes dystonia and oromandibular dystonia
Other Adverse Drug Reactions Observed During the Clinical Trial Evaluation of RISVAN
Other adverse reactions of <2% incidence but greater than placebo are shown below. The following list does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) which are part of the disease state, 3) for which a drug cause was remote, 4) which were so general as to be uninformative, or 5) which were not considered to have significant clinical implications.
General Disorders and Administration Site Conditions: asthenia, fatigue, injection site reaction (including injection site erythema, swelling and discomfort)
Metabolism and Nutrition Disorders: diabetes mellitus, increased appetite
Nervous System Disorders: dysarthria, dyskinesia, drooling, mental impairment, restless legs syndrome
Psychiatric Disorders: anorgasmia, enuresis, restlessness, tension
Renal and Urinary disorders: glycosuria, micturition urgency
Reproductive System and Breast Disorders: amenorrhea, dysmenorrhea, erectile dysfunction, galactorrhea
Vascular Disorders: hypotension, orthostatic hypotension
Additional Adverse Reactions Reported at an Incidence of 2% or more in Oral Risperidone-treated Adult Patients and Greater than Placebo
The following is a list of adverse reactions that are not reported in Table 3 above for RISVAN and occurred at an incidence of 2% or more in oral risperidone-treated adult patients and greater than placebo during the clinical trial evaluation of oral risperidone:
Eye Disorders: blurred vision
Gastrointestinal Disorders: nausea, dyspepsia, dry mouth, abdominal discomfort, salivary hypersecretion, diarrhea
General Disorders: fatigue, chest pain, asthenia
Infections and Infestations: upper respiratory tract infection, sinusitis, urinary tract infection
Investigations: heart rate increased
Musculoskeletal and Connective Tissue Disorders: back pain, arthralgia, pain in extremity
Nervous System Disorders: parkinsonism (includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia, masked facies, muscle rigidity, and Parkinson’s disease), tremor (includes tremor and parkinsonian rest tremor), dizziness postural
Psychiatric Disorders: insomnia, anxiety
Respiratory, Thoracic and Mediastinal Disorders: nasal congestion, dyspnea, epistaxis
Skin and Subcutaneous Tissue Disorders: rash, dry skin
Other Adverse Reactions Reported in Clinical Trials with Oral Risperidone
The following is a list of additional adverse reactions that have been reported during the clinical trial evaluation of oral risperidone, regardless of frequency of occurrence:
Blood and Lymphatic System Disorders: anemia, granulocytopenia, neutropenia
Cardiac Disorders: tachycardia, sinus bradycardia, sinus tachycardia, atrioventricular block first degree, bundle branch block left, bundle branch block right, atrioventricular block
Ear and Labyrinth Disorders: ear pain, tinnitus
Eye Disorders: vision blurred, oculogyration, ocular hyperemia, eye discharge, conjunctivitis, eye rolling, eyelid edema, eye swelling, eyelid margin crusting, dry eye, lacrimation increased, photophobia, glaucoma, visual acuity reduced
Gastrointestinal Disorders: dysphagia, fecaloma, fecal incontinence, gastritis, lip swelling, cheilitis, aptyalism
General Disorders: edema peripheral, thirst, gait disturbance, chest pain, influenza-like illness, pitting edema, edema, chills, sluggishness, malaise, face edema, discomfort, generalized edema, drug withdrawal syndrome, peripheral coldness, feeling abnormal
Immune System Disorders: drug hypersensitivity
Infections and Infestations: nasopharyngitis, upper respiratory tract infection, sinusitis, urinary tract infection, pneumonia, influenza, ear infection, viral infection, pharyngitis, tonsillitis, bronchitis, eye infection, localized infection, cystitis, cellulitis, otitis media, onychomycosis, acarodermatitis, bronchopneumonia, respiratory tract infection, tracheobronchitis, otitis media chronic
Investigations: body temperature increased, alanine aminotransferase increased, electrocardiogram abnormal, heart rate increased, eosinophil count increased, white blood cell count decreased, blood glucose increased, hemoglobin decreased, blood creatine phosphokinase increased, hematocrit decreased, body temperature decreased, blood pressure decreased, transaminases increased
Metabolism and Nutrition Disorders: decreased appetite, polydipsia, anorexia
Musculoskeletal, Connective Tissue, and Bone Disorders: joint stiffness, joint swelling, musculoskeletal chest pain, posture abnormal, myalgia, neck pain, muscular weakness, muscle rigidity, muscle contracture, rhabdomyolysis
Nervous System Disorders: balance disorder, dizziness postural, disturbance in attention, unresponsive to stimuli, depressed level of consciousness, movement disorder, hypokinesia, bradykinesia, transient ischemic attack, coordination abnormal, cerebrovascular accident, masked facies, speech disorder, syncope, loss of consciousness, hypoesthesia, tardive dyskinesia, muscle contractions involuntary, Parkinson’s disease, tongue paralysis, akinesia, cerebral ischemia, cerebrovascular disorder, neuroleptic malignant syndrome, diabetic coma, head titubation
Psychiatric Disorders: agitation, blunted affect, confusional state, middle insomnia, nervousness, sleep disorder, listlessness, libido decreased, anorgasmia
Renal and Urinary Disorders: enuresis, dysuria, pollakiuria, urinary incontinence
Reproductive System and Breast Disorders: menstrual irregular, gynecomastia, vaginal discharge, menstrual disorder, erectile dysfunction, retrograde ejaculation, ejaculation disorder, sexual dysfunction, breast enlargement
Respiratory, Thoracic, and Mediastinal Disorders: nasal congestion, dyspnea, epistaxis, wheezing, pneumonia aspiration, sinus congestion, dysphonia, productive cough, pulmonary congestion, respiratory tract congestion, rales, respiratory disorder, hyperventilation, nasal edema
Skin and Subcutaneous Tissue Disorders: rash, dry skin, erythema, skin discoloration, skin lesion, pruritus, skin disorder, rash erythematous, rash papular, acne, hyperkeratosis, seborrheic dermatitis, rash generalized, rash maculopapular
Vascular Disorders: flushing
Dose Dependent Adverse Reactions in Clinical Trials
Increased Prolactin
In the 12-week double-blind, placebo-controlled study, there was an increase in mean prolactin levels in fasting blood samples from baseline to end of the study in both the RISVAN 75 mg and 100 mg groups, while mean prolactin for the placebo group decreased during the study. Changes in mean prolactin were dose-dependent. See Table 3 for the percentage of RISVAN-treated patients with hyperprolactinemia with an incidence of greater than or equal to 2% and greater than placebo.
Extrapyramidal Symptoms (EPS)
Several methods were used to measure EPS, including: (1) the Barnes Akathisia Rating Scale (BARS) global clinical rating score which evaluates akathisia, (2) the Abnormal Involuntary Movement Scale (AIMS) scores which evaluates dyskinesia, (3) the Simpson-Angus Scale (SAS) global score which broadly evaluates parkinsonism, and (4) the incidence of spontaneous reports of EPS-related adverse reactions.
In the 12-week double-blind, placebo-controlled study, the mean changes from baseline in BARS, AIMS, and SAS total scores were comparable between RISVAN- and placebo-treated patients. At all postbaseline assessments, mean changes from baseline were between 0.0 and 0.1 (inclusive) for the BARS, between 0.1 and -0.1(inclusive) for the AIMS, and between 0.1 and 0.2 (inclusive) for the SAS.
In the 12-week double-blind, placebo-controlled study, there was a higher incidence of akathisia in RISVAN 100 mg (8%) compared with the RISVAN 75 mg (4%) and the placebo group (2%); reports of extrapyramidal disorders were higher in the RISVAN 100 mg group (12%) compared with the RISVAN 75 mg (8%) and the placebo group (3%).
Dystonia
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Injection Site Reactions with RISVAN
Local injection site pain was assessed using a subject-reported VAS scale (0 = no pain to 10 = unbearably painful) administered approximately 1 hour after each injection. In the 12-week, double-blind placebo-controlled study, the mean subject-reported injection site pain VAS scores were similar for all treatment groups after each of the three injections. Median VAS scores were 1 for all treatment groups after each of the three injections. In the 12-month, long-term safety study, the injection site pain VAS scores were highest on day 1 (mean of 1.8) and tended to lessen over time (mean of 1.5 at day 337).
The most commonly reported injection site related adverse reaction was pain. Throughout the double-blind placebo-controlled study and the long-term safety study, 14 out of 386 patients (4%) reported 18 cases of injection site pain after 2,827 injections (1%) of RISVAN. Of the 18 cases of injection site pain, 15 were rated as mild, and 3 were rated as moderate. Less common injection site adverse reactions were swelling (n=3, 1%), erythema (n=1, 0%), and discomfort (n=1, 0%), with all cases rated as mild in severity.
The local injection site was assessed by site investigators. Throughout the double-blind placebo-controlled study (n=290 receiving RISVAN), 7% of patients had redness, 2% had swelling, and 1% had induration. Throughout the long-term safety study (n=215 receiving RISVAN), 3% of patients had redness, 0.5% had swelling, and no patients had induration.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of oral risperidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions include: alopecia, anaphylactic reaction, angioedema, atrial fibrillation, cardiopulmonary arrest, catatonia, diabetic ketoacidosis in patients with impaired glucose metabolism, dysgeusia, hypoglycemia, hypothermia, ileus, inappropriate antidiuretic hormone secretion, intestinal obstruction, jaundice, mania, pancreatitis, pituitary adenoma, precocious puberty, pulmonary embolism, QT prolongation, sleep apnea syndrome, somnambulism, Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), sudden death, thrombocytopenia, thrombotic thrombocytopenic purpura, urinary retention, and water intoxication.
Postmarketing cases of extrapyramidal symptoms (dystonia and dyskinesia) have been reported in patients concomitantly taking methylphenidate and risperidone when there was an increase or decrease in dosage, initiation, or discontinuation of either or both medications.
7 DRUG INTERACTIONS
The interactions of RISVAN with coadministration of other drugs have not been evaluated. The drug interaction data provided in this section is based on studies with oral risperidone.
7.1 Drugs Having Clinically Important Interactions with RISVAN
Clinically significant drug interactions with RISVAN are shown in Table 4.
| Strong CYP2D6 Inhibitors | |
| Clinical Impact: | Concomitant use of RISVAN with strong CYP2D6 inhibitors may increase the plasma exposure of risperidone and lower the plasma exposure of a major active metabolite, 9-hydroxyrisperidone [see Clinical Pharmacology ( 12.3)]. |
| Intervention: | When used concomitantly with strong CYP2D6 inhibitors,the recommended dose of RISVAN is 75 mg started at least 4 weeks before the planned start of strong CYP2D6 inhibitors to adjust for the expected increase in plasma concentrations of risperidone. When strong CYP2D6 inhibitors are initiated in patients receiving RISVAN 75 mg, it is recommended to continue treatment with 75 mg unless clinical judgment necessitates interruption of RISVAN treatment. The effects of discontinuation of strong CYP2D6 inhibitors on the pharmacokinetics of risperidone and 9-hydroxyrisperidone have not been studied [see Clinical Pharmacology ( 12.3) ]. |
| Strong CYP3A4 Inducers | |
| Clinical Impact: | Concomitant use of RISVAN and a strong CYP3A4 inducer may cause decreases in the combined plasma concentrations of risperidone and 9- hydroxyrisperidone which could lead to decreased efficacy of RISVAN [see Clinical Pharmacology ( 12.3)]. |
| Intervention: | Changes in efficacy and safety should be carefully monitored with any dose adjustment of RISVAN. At the initiation of therapy with a strong CYP3A4 inducer, patients should be closely monitored during the first 4 to 8 weeks. In patients receiving RISVAN 75 mg, increase the dose to 100 mg. In patients receiving RISVAN 100 mg, additional oral risperidone therapy may need to be considered. On discontinuation of a strong CYP3A4 inducer, the dosage of RISVAN or any additional oral risperidone therapy should be reevaluated and, if necessary, decreased to adjust for the expected increase in plasma concentration of risperidone and 9-hydroxyrisperidone. For patients treated with RISVAN 75 mg and discontinuing from a strong CYP3A4 inducer, it is recommended to continue treatment with the 75 mg dose unless clinical judgment necessitates interruption of RISVAN treatment [see Dosage and Administration (2.4)]. |
| Centrally-Acting Drugs and Alcohol | |
| Clinical Impact: | Due to additive pharmacologic effects, the concomitant use of centrally-acting drugs, including alcohol, may increase nervous system disorders. |
| Intervention: | Caution should be used when RISVAN is administered in combination with other centrally-acting drugs or alcohol. |
| Hypotensive Agents | |
| Clinical Impact: | Because of its potential for inducing hypotension, RISVAN may enhance the hypotensive effects of other therapeutic agents with this potential. |
|
Intervention: | Caution should be used when RISVAN is administered in combination with other therapeutic agents with hypotensive effects. |
| Dopamine Agonists | |
| Clinical Impact: | Agents with central antidopaminergic activity such as RISVAN may antagonize the pharmacologic effects of dopamine agonists. |
|
Intervention: | Caution should be used when RISVAN is administered in combination with levodopa and dopamine agonists. |
| Methylphenidate | |
| Clinical Impact: |
Concomitant use with methylphenidate, when there is change in dosage of either medication, may increase the risk of extrapyramidal symptoms (EPS) [see Adverse Reactions (6.2)]. |
| Intervention: | Monitor for symptoms of EPS with concomitant use of RISVAN and methylphenidate. |
7.2 Drugs Having No Clinically Important Interactions with RISVAN
Based on pharmacokinetic studies with oral risperidone, no dosage adjustment of RISVAN is required when administered concomitantly with amitriptyline, cimetidine, ranitidine, clozapine, topiramate and moderate CYP3A4 inhibitors (erythromycin). Additionally, no dosage adjustment is necessary for lithium, valproate, topiramate, digoxin and CYP2D6 substrates (donepezil and galantamine) when coadministered with RISVAN [see Clinical Pharmacology ( 12.3)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including RISVAN, during pregnancy. Healthcare providers are encouraged to advise patients to register by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at http://womensmentalhealth.org/clinical-andresearch-programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall available data from published epidemiologic studies of pregnant women exposed to risperidone have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia and with exposure to antipsychotics, including RISVAN, during pregnancy (see Clinical Considerations).
Oral administration of risperidone to pregnant mice caused cleft palate at doses 3 to 4 times the maximum recommended human oral dose (MRHD) of 16 mg/day with maternal toxicity observed at 4-times MRHD based on mg/m 2 body surface area. Risperidone did not cause malformations in rats or rabbits when orally administered risperidone at doses up to 6-times the oral MRHD based on mg/m 2 body surface area. Increased stillbirths and decreased birth weight occurred after oral risperidone administration to pregnant rats at 1.5-times the oral MRHD based on mg/m 2 body surface area. Learning was impaired in offspring of rats when the dams were dosed at 0.6-times the oral MRHD and offspring mortality increased at doses 0.1 to 3 times the oral MRHD based on mg/m 2 body surface area.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
There is a risk to the mother from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide. Schizophrenia is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs, including risperidone, during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A prospective observational study including 6 women treated with risperidone, demonstrated placental passage of risperidone and paliperidone. A retrospective cohort study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects. There was a small increase in the risk of major birth defects (RR=1.26, 95% CI 1.02-1.56) and of cardiac malformations (RR=1.26, 95% CI 0.88-1.81) in a subgroup of 1566 women exposed to risperidone, during the first trimester of pregnancy; however, there is no mechanism of action to explain the difference in malformation rates.
Animal Data
No developmental toxicity studies were conducted with RISVAN.
Oral administration of risperidone to pregnant mice during organogenesis caused cleft palate at 10 mg/kg/day which is 3-times the oral MRHD of 16 mg/day based on mg/m 2 body surface area; maternal toxicity occurred at 4-times the oral MRHD. Risperidone did not cause malformations when administered orally to rats at 0.6 to 10 mg/kg/day and rabbits at 0.3 to 5 mg/kg/day, which are up to 6-times the oral MRHD based on mg/m 2 body surface area. Learning was impaired in offspring of rats dosed orally throughout pregnancy at 1 mg/kg/day which is 0.6-times the oral MRHD and neuronal cell death increased in fetal brains of offspring of rats dosed during pregnancy at 1 and 2 mg/kg/day which are 0.6 and 1.2-times the oral MRHD based on mg/m 2 body surface area; postnatal development and growth of the offspring were also delayed.
Rat offspring mortality increased during the first 4 days of lactation when pregnant rats were dosed throughout gestation at 0.16 to 5 mg/kg/day which are 0.1-to 3-times the oral MRHD based on mg/m 2 body surface area. It is not known whether these deaths were due to a direct effect on the fetuses or pups or to effects on the dams; a no-effect dose could not be determined. The rate of stillbirths was increased at 2.5 mg/kg or 1.5-times the oral MRHD based on mg/m 2 body surface area.
In a rat cross-fostering study the number of live offspring was decreased, the number of stillbirths increased, and the birth weight was decreased in offspring of drug-treated pregnant rats. In addition, the number of deaths increased by Day 1 among offspring of drug-treated pregnant rats, regardless of whether or not the offspring were cross-fostered. Risperidone also appeared to impair maternal behavior in that offspring body weight gain and survival (from Day 1 to 4 of lactation) were reduced in offspring born to control but reared by drug-treated dams. All of these effects occurred at 5 mg/kg which is 3-times the oral MRHD based on mg/m 2 and the only dose tested in the study.
8.2 Lactation
Risk Summary
Limited data from published literature report the presence of risperidone and its metabolite, 9-hydroxyrisperidone, in human breast milk at relative infant dose ranging between 2.3% and 4.7% of the maternal weight-adjusted dosage. There is no information on the effects on milk production; however, there are reports of sedation, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) in breastfed infants exposed to risperidone (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RISVAN and any potential adverse effects on the breastfed child from RISVAN or from the mother’s underlying condition.
Clinical Considerations
Infants exposed to RISVAN through breastmilk should be monitored for excess sedation, failure to thrive, jitteriness, and extrapyramidal symptoms tremors and abnormal muscle movements).
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the pharmacologic action of risperidone (D2 receptor antagonism), treatment with RISVAN may result in an increase in serum prolactin levels, which may lead to a reversible reduction in fertility in females of reproductive potential [see Warnings and Precautions (5.7)].
8.4 Pediatric Use
The safety and effectiveness of RISVAN have not been established in pediatric patients.
Juvenile Animal Toxicity Data
No juvenile animal studies were conducted with intramuscular risperidone suspension.
Juvenile dogs were treated with oral risperidone from weeks 10 to 50 of age (equivalent to the period of childhood through adolescence in humans), at doses of 0.31, 1.25, or 5 mg/kg/day. Bone length and density were decreased with a no-effect dose of 0.31 mg/kg/day. In addition, sexual maturation was delayed at all doses in both males and females. The above effects showed little or no reversibility in females after a 12 week drug-free recovery period.
Juvenile rats, treated with oral risperidone from days 12 to 50 of age (equivalent to the period of infancy through adolescence in humans) showed impaired learning and memory performance (reversible only in females), with a no-effect dose of 0.63 mg/kg/day. No other consistent effects on neurobehavioral or reproductive development were seen up to the highest tested dose of 1.25 mg/kg/day.
8.5 Geriatric Use
Clinical studies of RISVAN in the treatment of schizophrenia did not include patients older than 65 years to determine whether they respond differently from younger patients.
In general, dose selection for geriatric patients should be cautious, usually starting at the low end of the dosing range, reflecting a decreased pharmacokinetic clearance in geriatric patients, as well as a greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Risperidone is substantially excreted by the kidneys, and the risk of reactions may be greater in patients with impaired renal function. Because geriatric patients are more likely to have decreased renal function, care should be taken in dose selection for RISVAN, and it may be useful to monitor renal function [see Dosage and Administration (2.2), Warnings and Precautions (5.7), and Clinical Pharmacology (12.3)].
Elderly patients with dementia-related psychosis treated with RISVAN are at an increased risk of death compared to placebo. RISVAN is not approved for the treatment of patients with dementia related psychosis [see Boxed Warning and Warnings and Precautions (5.1, 5.2)].
8.6 Renal Impairment
In patients with renal impairment, titrate with oral risperidone (up to at least 3 mg) before initiating treatment with RISVAN at a dose of 75 mg [see Dosage and Administration (2.3)].
RISVAN was not studied in patients with renal impairment; however, such effect has been investigated with oral risperidone [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In patients with hepatic impairment, titrate with oral risperidone (up to at least 3 mg) before initiating treatment with RISVAN at a dose of 75 mg [see Dosage and Administration (2.3)].
RISVAN was not studied in patients with hepatic impairment; however, such effect has been investigated with oral risperidone [see Clinical Pharmacology (12.3)].
8.8 Patients with Parkinson’s Disease or Lewy Body Dementia
Patients with Parkinson’s Disease or Dementia with Lewy Bodies can experience increased sensitivity to RISVAN. Manifestations can include confusion, obtundation, postural instability with frequent falls, extrapyramidal symptoms, and clinical features consistent with neuroleptic malignant syndrome.
10 OVERDOSAGE
Human Experience
Premarketing experience with oral risperidone included eight reports of overdosage with estimated doses ranging from 20 to 300 mg and no fatalities. In general, reported signs and symptoms were those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. One case, involving an estimated overdose of 240 mg, was associated with hyponatremia, hypokalemia, prolonged QT, and widened QRS. Another case, involving an estimated overdose of 36 mg, was associated with a seizure.
Postmarketing experience includes reports of acute oral risperidone overdosage, with estimated doses of up to 360 mg. In general, the most frequently reported signs and symptoms are those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. Other adverse reactions reported since market introduction related to oral risperidone overdose include prolonged QT interval and convulsions. Torsade de pointes has been reported in association with combined overdose of oral risperidone and paroxetine.
Management of Overdosage
There is no specific antidote to risperidone. Provide supportive care including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs.
The pharmacokinetic profile of RISVAN should be considered when treating patients with overdose.
Consider contacting the Poison Help Line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
11 DESCRIPTION
RISVAN contains risperidone, an atypical antipsychotic. Risperidone belongs to the chemical class of benzisoxazole derivatives. The chemical designation 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl) piperidin-1-yl] ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a] pyrimidin-4-one. Its molecular formula is C23H27FN4O2 and its molecular weight is 410.5 g/mol.
The structural formula is:
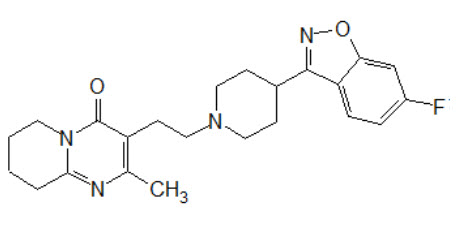
Risperidone is a white to off-white powder. It is practically insoluble in water and soluble in methanol and 0.1 N HCl.
RISVAN is available as a sterile two-syringe mixing system; a solvent syringe prefilled with the solvent dimethyl sulfoxide, a transparent and colorless solution. The powder syringe is prefilled with risperidone and poly (lactide-co-glycolide) acid co-polymer. The powder is white to white-yellowish in color.
RISVAN is available as an extended-release injectable suspension, for intramuscular use, in the following strengths of risperidone: 75 mg and 100 mg.
| Component | RISVAN 75 mg | RISVAN 100 mg |
| Risperidone | 75 mg | 100 mg |
| PLGA | 150 mg | 200 mg |
| Dimethylsulfoxide | 350 mg | 466.7 mg |
PLGA Poly (D,L-lactide-co-glycolide) 50:50
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of risperidone, in schizophrenia, is unclear. The drug’s therapeutic activity in schizophrenia could be mediated through a combination of dopamine Type 2 (D 2) and serotonin Type 2 (5HT 2) receptor antagonism. The clinical effect from risperidone results from the combined concentrations of risperidone and its major metabolite, 9-hydroxyrisperidone (paliperidone) [see Clinical Pharmacology ( 12.3)] . Antagonism at receptors other than D 2 and 5HT 2 may explain some of the other effects of risperidone.
12.2 Pharmacodynamics
Risperidone is a monoaminergic antagonist with high affinity (Ki of 0.12 to 7.3 nM) for the serotonin Type 2 (5HT 2), dopamine Type 2 (D 2), α1 and α2 adrenergic, and H 1 histaminergic receptors. Risperidone showed low to moderate affinity (Ki of 47 to 253 nM) for the serotonin 5HT 1C, 5HT 1D, and 5HT 1A receptors, weak affinity (Ki of 620 to 800 nM) for the dopamine D 1 and haloperidol-sensitive sigma site, and no affinity (when tested at concentrations > 10 -5 M) for cholinergic muscarinic or β1 and β2 adrenergic receptors.
12.3 Pharmacokinetics
Following single doses of RISVAN, plasma exposure (AUC and Cmax) of risperidone, 9-hydroxyrisperidone, and total active moiety (risperidone+9-hydroxyrisperidone) increased in an approximately dose proportional manner over the dose range of 25 mg (0.25 times the maximum recommended dose of RISVAN) to 100 mg. Steady state minimum (Cmin) and peak (Cmax) plasma concentrations of risperidone, 9-hydroxyrisperidone and total active moiety were reached by the first and second injections, respectively. Based on Cmin and Cmax values for total active moiety, the average accumulation ratios were approximately 1.3-fold and 1.8-fold, respectively.
The average plasma concentrations (Cavg) of total active moiety were 22 ng/mL and 28 ng/mL for 3 mg oral risperidone and 75 mg RISVAN, respectively. The Cavg of total active moiety were 29 ng/mL and 37 ng/mL for 4 mg oral risperidone and 100 mg RISVAN, respectively.
Absorption
RISVAN contains risperidone in a suspension delivery system. Following intramuscular (IM) injection, it forms a depot that provides sustained plasma levels over the monthly interval.
Following IM injection, RISVAN shows two absorption peaks for risperidone, 9-hydroxyrisperidone and total active moiety in plasma. The initial peak occurs between 24 h to 48 h and the second peak occurs between Day 18 to Day 25.
Following repeated IM injection of RISVAN 75 mg in the deltoid muscle, on average, 22% higher Cmax was observed compared with injection in the gluteal muscle. The average concentration (Cavg) at steady state was similar for both injection sites.
Distribution
The volume of distribution of risperidone is 1-2 L/kg. In plasma, risperidone is bound to albumin and alpha1-acid glycoprotein. The plasma protein binding of risperidone and 9-hydroxyrisperidone is 90% and 77%, respectively. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites.
Elimination
Metabolism
Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme cytochrome CYP2D6 with minor contribution by CYP3A4. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone.
CYP2D6, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP2D6 is subject to genetic polymorphism (about 6 to 8% of Caucasians, and a very low percentage of Asians, have little or no activity and are “poor metabolizers”) and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP2D6 metabolizers convert it much more slowly. Plasma exposures to total active moiety were similar in CYP2D6 extensive, intermediate and poor metabolizers following intramuscular injection with RISVAN.
Excretion
Risperidone and its metabolites are eliminated via the urine and, to a much lesser extent, via the feces. As illustrated by a mass balance study of a single 1 mg oral dose of 14C-risperidone administered as solution to three healthy male volunteers, total recovery of radioactivity at 1 week was 84%, including 70% in the urine and 14% in the feces.
Following a single injection of RISVAN, the mean half-life (T1/2) ranged from 3.9 to 7.5 days for risperidone, from 8.1 to 8.2 days for 9-hydroxyrisperidone, and from 7.1 to 8.7 days for the active moiety.
Specific Populations
Based on population pharmacokinetic analyses, age, sex and race do not have a clinically meaningful effect on the pharmacokinetics of RISVAN.
Patients with Renal Impairment
RISVAN was not studied in patients with renal impairment, however, in patients with moderate to severe renal disease treated with oral risperidone, the apparent clearance (CL/F) of total active moiety was decreased by 60% in patients with moderate to severe renal disease compared with young healthy subjects [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
RISVAN was not studied in patients with hepatic impairment. In studies with oral risperidone, the pharmacokinetics of subjects with liver disease were comparable to those in young healthy subjects; the mean free fraction of risperidone in plasma was increased by about 35% because of the diminished concentration of both albumin and α1-acid glycoprotein [see Use in Specific Populations (8.4)].
Drug Interaction Studies
No specific drug interaction studies have been performed with RISVAN. The drug interaction data provided in this section is based on studies with oral risperidone. Effects of other drugs on the exposures of risperidone, 9-hydroxyrisperidone and total active moiety as well as the effects of risperidone on the exposures of other drugs are summarized below.
Clinical studies
Effects of Other Drugs on Risperidone, 9-hydroxyrisperidone and Total Active Moiety Pharmacokinetics
Strong CYP2D6 Inhibitors (Fluoxetine and Paroxetine)
Fluoxetine (20 mg once daily) and paroxetine (20 mg once daily), potent CYP2D6 inhibitors, have been shown to increase the plasma concentration of risperidone by 2.5- to 2.8-fold and 3- to 9-fold, respectively. Fluoxetine did not affect the plasma concentration of 9-hydroxyrisperidone. Paroxetine lowered the concentration of 9-hydroxyrisperidone by about 10%. The effects of discontinuation of concomitant fluoxetine or paroxetine therapy on the pharmacokinetics of risperidone and 9hydroxyrisperidone have not been studied.
Moderate CYP3A4 Inhibitor (Erythromycin)
There were no significant interactions between oral risperidone and erythromycin, a moderate CYP3A4 inhibitor.
Strong CYP3A4 Inducer (Carbamazepine)
Carbamazepine co-administration with oral risperidone decreased the steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone by about 50%. Plasma concentrations of carbamazepine did not appear to be affected. Co-administration of other known CYP3A4 enzyme inducers (e.g., phenytoin, rifampin, and phenobarbital) with risperidone may cause similar decreases in the combined plasma concentrations of risperidone and 9-hydroxyrisperidone.
Amitriptyline, Cimetidine, Ranitidine, Clozapine, Topiramate
Clinically meaningful pharmacokinetic interaction between RISVAN and other drugs, such as amitriptyline, cimetidine, ranitidine and clozapine, is not expected.
- Amitriptyline did not affect the pharmacokinetics of risperidone or of risperidone and 9hydroxyrisperidone combined following concomitant administration with oral risperidone.
- Cimetidine and ranitidine increased the bioavailability of oral risperidone by 64% and 26%, respectively. However, cimetidine did not affect the AUC of risperidone and 9hydroxyrisperidone combined, whereas ranitidine increased the AUC of risperidone and 9hydroxyrisperidone combined by 20%.
- Chronic administration of clozapine with oral risperidone have shown to affect the clearance of risperidone, however, clinical relevance is unknown.
- There was no clinically relevant effect of oral risperidone (1 to 6 mg/day) on the pharmacokinetics of topiramate 400 mg/day.
Effects of Oral Risperidone on Pharmacokinetics of Other Drugs
Lithium
Repeated doses of oral risperidone (3 mg twice daily) did not affect the exposure (AUC) or peak plasma concentrations (Cmax) of lithium (n = 13).
Valproate
Repeated doses of oral risperidone (4 mg once daily) did not affect the pre-dose or average plasma concentrations and exposure (AUC) of valproate (1000 mg/day in three divided doses) compared to placebo (n = 21). However, there was a 20% increase in valproate peak plasma concentration (Cmax) after concomitant administration of oral risperidone.
Topiramate
Oral risperidone administered at doses from 1 to 6 mg/day concomitantly with topiramate 400 mg/day resulted in a 23% decrease in risperidone Cmax and a 33% decrease in risperidone AUC0-12 hour at steady state. Minimal reductions in the exposure to risperidone and 9-hydroxyrisperidone combined, and no change for 9-hydroxyrisperidone were observed. This interaction is unlikely to be of clinical significance. There was no clinically relevant effect of oral risperidone on the pharmacokinetics of topiramate.
Digoxin
Oral risperidone (0.25 mg twice daily) did not show a clinically relevant effect on the pharmacokinetics of digoxin.
CYP2D6 Substrates (Donepezil and Galantamine)
In drug interaction studies, oral risperidone did not significantly affect the pharmacokinetics of donepezil and galantamine, which are metabolized by CYP2D6.
In vitro studies
In vitro studies indicate that risperidone is a relatively weak inhibitor of CYP2D6. Therefore, RISVAN is not expected to substantially inhibit the clearance of drugs that are metabolized by this enzymatic pathway.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies were conducted with RISVAN.
Carcinogenicity studies were conducted with oral risperidone in mice and rats. Risperidone was administered in the diet at doses of 0.63, 2.5, and 10 mg/kg for 18-months to mice and for 25-months to rats. These doses are equivalent to approximately 0.2-, 0.75-, and 3-times (mice) and 0.4-, 1.5-, and 6-times (rats) the oral MHRD of 16 mg/day, based on a mg/m 2 body surface area. A maximum tolerated dose was not achieved in male mice. There were statistically significant increases in pituitary gland adenomas, endocrine pancreas adenomas, and mammary gland adenocarcinomas. The table below summarizes the multiples of the human oral dose on a mg/m 2 (mg/kg) basis at which these tumors occurred.
| Multiples of Maximum Human Oral Dose in mg/m 2 (mg/kg) | ||||
| Tumor Type | Species | Sex | Lowest Effect Level | Highest No-Effect Level |
| Pituitary adenomas | mouse | Female | 0.75 (9.4) | 0.2 (2.4) |
| Endocrine pancreas adenomas | rat | Male | 1.5 (9.4) | 0.4 (2.4) |
| Mammary gland adenocarcinomas | mouse | Female | 0.2 (2.4) | none |
| rat | Female | 0.4 (2.4) | none | |
| rat | Male | 6.0 (37.5) | 1.5 (9.4) | |
| Mammary gland neoplasm, Total | rat | Male | 1.5 (9.4) | 0.4 (2.4) |
Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum prolactin levels were not measured during the oral risperidone carcinogenicity studies; however, measurements during subchronic toxicity studies showed that oral risperidone elevated serum prolactin levels 5-to 6-fold in mice and rats at the same doses used in the carcinogenicity studies. Serum prolactin levels increased in a dose-dependent manner up to 6- and 1.5-fold in male and female rats, respectively, at the end of the 24-month treatment with IM risperidone microspheres every 2 weeks. An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be prolactin-mediated. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unclear [see Warnings and Precautions (5.7)].
Mutagenesis
No evidence of mutagenic or clastogenic potential for risperidone was found in the in vitro tests of Ames gene mutation, the mouse lymphoma assay, rat hepatocyte DNA-repair assay, the chromosomal aberration test in human lymphocytes, Chinese hamster ovary cells, or in the in vivo oral micronucleus test in mice and the sex-linked recessive lethal test in Drosophila.
In addition, no evidence of mutagenic potential was found in the in vitro Ames reverse mutation test for RISVAN.
Impairment of Fertility
No mating and fertility studies were conducted with RISVAN.
Oral risperidone (0.16 to 5 mg/kg) impaired mating, but not fertility, in rat reproductive studies at doses 0.1-to 3-times the oral maximum recommended human dose (MRHD), of 16 mg/day based on mg/m2 body surface area. The effect appeared to be in females, since impaired mating behavior was not noted in the male fertility study. In a subchronic study in Beagle dogs in which risperidone was administered orally at doses of 0.31 to 5 mg/kg, sperm motility and concentration were decreased at doses 0.6-to 10-times the oral MRHD based on mg/m2 body surface area. Dose-related decreases were also noted in serum testosterone at the same doses. Serum testosterone and sperm parameters partially recovered, but remained decreased after treatment was discontinued. A no-effect dose could not be determined in either rat or dog.
14 CLINICAL STUDIES
Efficacy of RISVAN in the treatment of schizophrenia in adults is based upon adequate and well-controlled studies of oral risperidone as well as on one 12-week, randomized, double-blind, placebo-controlled study with RISVAN in adult patients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for schizophrenia (Study 1; NCT 03160521).
Study 1 evaluated the efficacy of RISVAN (75 mg and 100 mg intramuscular every 4 weeks) compared with placebo in adults (age 18 to 65 years, inclusive) experiencing acute exacerbations of schizophrenia. Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score of 80 to 120, inclusive (moderate to severely ill) at the screening visit, occurring 1 to 8 days before the start of double-blind treatment, without an improvement in the PANSS total score of ≥ 20% between screening and the first dosing day.
At the screening visit, patients who had never taken risperidone received 2 mg daily of oral risperidone for 3 days to establish tolerability. Patients were admitted to an inpatient setting, where they remained for 1 to 14 days after the first intramuscular dose of study treatment, as clinically warranted. Patients were randomized to receive 3 doses of intramuscular RISVAN (75 mg or 100 mg) or placebo every 4 weeks (on Day 1, Day 29, and Day 57). No supplemental antipsychotic medications were permitted during the study treatment period.
The primary endpoint was the change in PANSS total score from baseline to end of study at Day 85. Both RISVAN 75- and 100-mg doses demonstrated a statistically significant improvement compared with placebo based on the primary endpoint (Table 7). The change in PANSS total score from baseline at each visit from baseline through Day 85 are displayed in Figure 1.
Characteristics of the patient population were balanced across the treatment groups. The mean baseline PANSS total score was approximately 96 in each group. Most patients were male (66% to 68% per group), and the mean ages were 41 to 43 years in each group. Approximately half of the patients were black or African-American (47% to 53% per group) and half were white (46% to 51% per group). A total of 390 patients were included in the primary efficacy population.
Subgroup analyses by gender and race did not suggest differences in response. All patients in Study 1 were less than 65 years of age.
| Treatment Group | Number
of Patients | Mean
Baseline Score (SD) | LS Mean
Change from Baseline (SE) | Placebo-subtracted
Difference a (95% CI) |
|
RISVAN 75 mg |
129 | 96.3 (8.47) |
-24.6 (1.51) |
-13.0 (-17.3 to -8.8) |
|
RISVAN 100 mg |
129 |
96.1 (8.42) |
-24.7 (1.54) |
-13.3 (-17.6 to -8.9) |
|
Placebo |
132 |
96.4 (7.21) |
-11.0 (1.56) |
--- |
|
PANSS: Positive and Negative Syndrome Scale; SD=standard deviation; SE=standard error; LS Mean=least‑squares mean; CI=confidence interval a Difference (drug minus placebo) in LS mean change from baseline |
||||
Figure 1. Change in Baseline in PANSS Total Score by Study Visit in Adults with Schizophrenia
(Study 1)
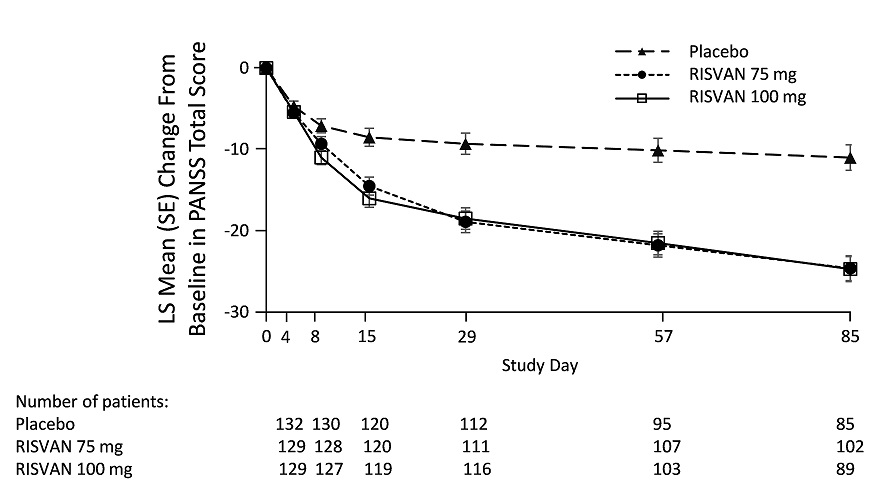
The secondary efficacy endpoint was defined as the mean change from baseline at Day 85 on the Clinical Global Impression – Severity (CGI-S) score. Both RISVAN treatment groups demonstrated statistically significantly better CGI-S scores versus placebo.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
RISVAN (risperidone) for extended-release injectable suspension is available in dosage strengths of 75 mg and 100 mg that is an off-white to off-white-yellowish, uniform suspension when fully mixed.
RISVAN 75 mg is supplied in a single-dose kit, packaged in a carton (NDC: 82090-001-01), containing the following:
- One pouch with a desiccant and a sterile syringe (labelled ‘R’) prefilled with white to white-yellowish powder of risperidone and poly (lactide-co-glycolide) acid co- polymer.
- One pouch with a desiccant and a sterile syringe (labelled ‘S’) prefilled with dimethyl sulfoxide solvent (transparent and colorless).
- Two administration needles, one 21G, 1 inch for deltoid and another 20G, 2 inch for gluteal administration.
RISVAN 100 mg is supplied in a single-dose kit, packaged in a carton (NDC: 82090-003-01), containing the following:
- One pouch with a desiccant and a sterile syringe (labelled ‘R’) prefilled with a white to white-yellowish powder of risperidone and poly (lactide-co-glycolide) acid co- polymer.
- One pouch with a desiccant and a sterile syringe (labelled ‘S’) prefilled with dimethyl sulfoxide solvent (transparent and colorless).
- Two administration needles, one 21G, 1 inch for deltoid and another 20G, 2 inch for gluteal administration.
Storage and Handling
Store at room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (between 59°F and 86°F) in the unopened original packaging.
17 PATIENT COUNSELING INFORMATION
Neuroleptic Malignant Syndrome (NMS)
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS) that has been reported in association with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the healthcare provider or report to the emergency room if they experience signs and symptoms of NMS [see Warnings and Precautions (5.3)].
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur [see Warnings and Precautions (5.4)].
Metabolic Changes
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus and the need for specific monitoring, including blood glucose, lipids, and weight [see Warnings and Precautions (5.5)].
Hyperprolactinemia
Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of RISVAN. Advise them to seek medical attention if they experience any of the following: amenorrhea or galactorrhea in females, erectile dysfunction, or gynecomastia in males [see Warnings and Precautions (5.6)].
Orthostatic Hypotension and Syncope
Educate patients about the risk of orthostatic hypotension and syncope, particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose [see Warnings and Precautions (5.7)].
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug-induced leukopenia/neutropenia that they should have their CBC monitored while being treated with RISVAN [see Warnings and Precautions (5.9)].
Potential for Cognitive and Motor Impairment
Inform patients that RISVAN has the potential to impair judgment, thinking, or motor skills. Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery, or operating a motor vehicle, until they are reasonably certain that RISVAN therapy does not affect them adversely [see Warnings and Precautions (5.10)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism [Warnings and Precautions (5.13)].
Heat Exposure and Dehydration
Educate patients regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.14)].
Concomitant Medication
Advise patients to inform their healthcare providers if they are taking, or plan to take, any prescription or over-the-counter drugs, as there is a potential for interaction [see Drug Interactions (7)].
Alcohol
Advise patients to avoid alcohol during treatment with RISVAN [see Drug Interactions (7.1)].
Pregnancy
Advise patients to notify their healthcare professional if they become pregnant or intend to become pregnant during treatment with RISVAN. Advise patients that RISVAN may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to RISVAN during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using RISVAN to monitor infants for somnolence, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that RISVAN may impair fertility due to an increase in serum prolactin levels. The effects on fertility are reversible [see Use in Specific Populations (8.3)].
Manufactured for:
Laboratorios Farmacéuticos Rovi S.A. Madrid, Spain
Powder syringe manufactured by Laboratorios Farmacéuticos Rovi S.A. Madrid, Spain
Solvent syringe manufactured by Rovi Pharma Industrial Services S.A.U. Madrid, Spain
INSTRUCTIONS FOR USE - 75 mg
RISVAN ®
(risperidone)
extended-release injectable suspension
75 mg, single dose
For intramuscular injection
Do not administer by any other route
Must be reconstituted before use
IMPORTANT INFORMATION
- To be administered by a healthcare professional only.
- Label the administration syringe immediately after reconstitution, then administer immediately.
- To be administered intramuscularly only.
- Two administration safety needles are included: one for deltoid injection (21G, 1 inch) and the other for gluteus injection (20G, 2 inch).
- Do not substitute any component of the drug kit.
- As universal precaution, always wear gloves.
- The drug kit should be stored at room temperature between 20ºC – 25ºC (68ºF – 77ºF).
- Read the complete directions before use.
1. CHECK CONTENTS
Working on a clean surface, open the pouches and discard the desiccant.
Each carton of RISVAN ® contains:
- One pouch with a RISVAN ® prefilled syringe with a WHITE plunger rod and WHITE finger flange.
- One pouch with SOLVENT for RISVAN ® prefilled syringe with a TRANSPARENT plunger rod and a RED finger flange.
- Two administration needles 21G, 1 inch for deltoid (green cap) and a 20G, 2 inch for gluteus (yellow cap).
 Discard the kit if any component is damaged.
Discard the kit if any component is damaged.
Parenteral drug products should always be visually inspected for particles and discoloration prior to administration whenever the solution and container permit it.
1.1 Inspect solvent syringe
ENSURE that SOLVENT syringe content flows normally as a liquid. The solvent freezes at 66 ºF. If the solvent is frozen or partially frozen, warm it at room temperature until it flows normally.
1.2. Dislodge powder syringe
TAP the RISVAN ® syringe to dislodge potential packed powder near the cap.
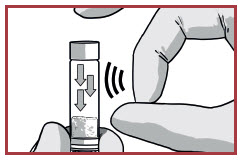
2. CONNECT THE SYRINGES
2.1. Uncap syringes in upright position
Hold both syringes in upright position to prevent loss of product. Figure A
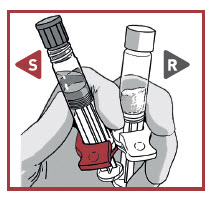
Figure A
PULL the cap off the Solvent syringe. Figure B
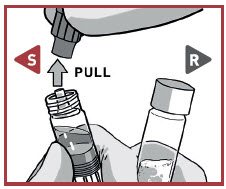
Figure B
TWIST and PULL the Powder syringe cap off. Figure C
 is in the upright position to prevent loss of product.
is in the upright position to prevent loss of product.
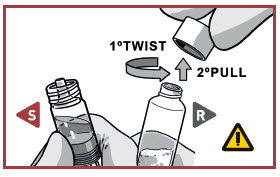
Figure C
2.2 Connect the syringes
Pick the solvent syringe S or slightly tilt it vertically.
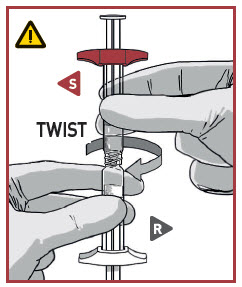
TWIST the syringes together until you feel a slight resistance.
 is in the upright position to prevent loss of product.
is in the upright position to prevent loss of product.
3. MIX THE CONTENTS
 STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
- PUSH VIGOROUSLY the Solvent content S towards the Powder syringe.
- DO NOT WAIT for powder wetting and QUICKLY start mixing contents by pushing the plungers FAST and alternately for 100 pushes (2 pushes within 1 second, approximately 1 minute).
- ENSUREmedication is passing between both syringes for proper mixing: medication is viscous and you will need to apply force when pressing on the plunger rods.
Mix for at least 100 pushes by doing alternately 1
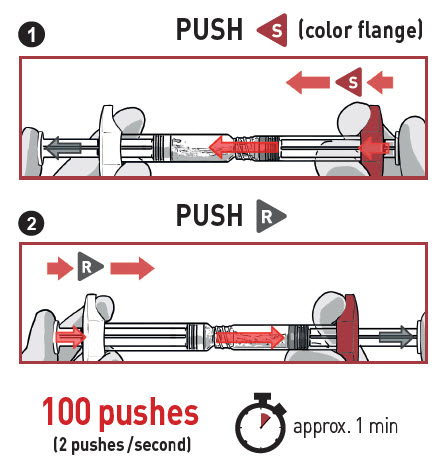
 Make sure medication is passing between both syringes
Make sure medication is passing between both syringes
When medication is correctly mixed, the appearance will be a uniform suspension off white to yellowish color andthick consistency.

 Proceed immediately to prepare the injection for administration
Proceed immediately to prepare the injection for administration
4. PREPARE INJECTION SYRINGE
4.1 Transfer medication
Place downward pressure on the R syringe that has the colored flange.
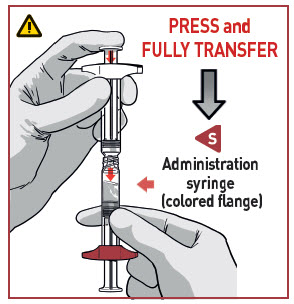
 Make sure all the content is transferred.
Make sure all the content is transferred.
4.2. Detach syringes
Once the medication is fully transferred, separate the two syringes by untwisting.
Proceed immediately to prepare the injection syringe for administration.
The injection must be given within 15 minutes after reconstitution.
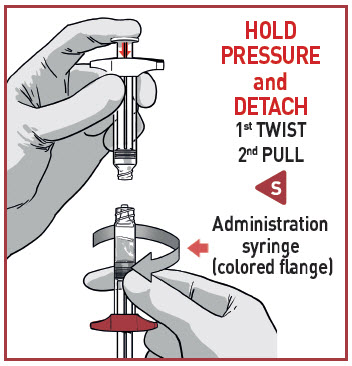
4.3. Attach the safety needle
Choose the proper needle:
- Deltoid: 21G, 1 inch for deltoid (green cap).
- Gluteus: 20G, 2 inch for gluteus (yellow cap).
Attach it using a clockwise twisting motion.
Do not over-tighten.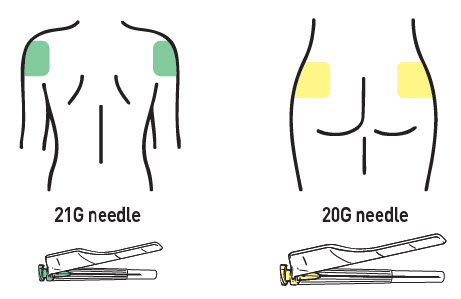
4.4 Remove excess air
Remove needle cover and push out the excess air (only big bubbles) from the syringe barrel.
 DO NOT expel any drops of medication
DO NOT expel any drops of medication
If you see the medication appearing at the needle tip, pull slightly back on the plunger to prevent medication spillage.
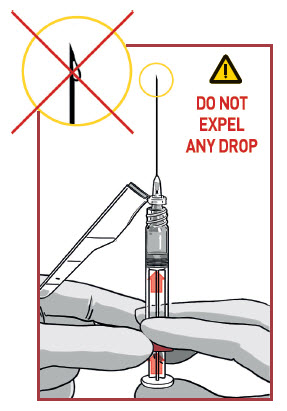
5. ADMINISTER AND DISPOSE
5.1 Inject medication
Insert the needle fully into the deltoid or gluteal muscle. DO NOT INJECT BY ANY OTHER ROUTE.
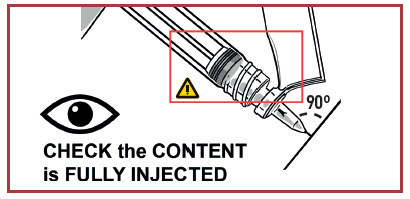
 WARNINGS
WARNINGS
- THICK MEDICATION, INJECT IT SLOWLY AND STEADILY. MAKE SURE TO FULLY INJECT IT.
- The injection time is longer than usual due to the viscosity of the medication.
- Wait a few seconds before removing the needle.
- Avoid inadvertent injection into a blood vessel.
5.2. Dispose of needle and syringe
Cover the needle by pressing on the needle guard using a finger or a flat surface and dispose immediately in a secure sharps disposal container.
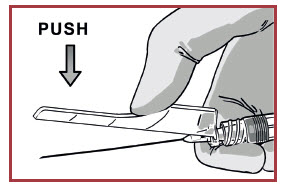
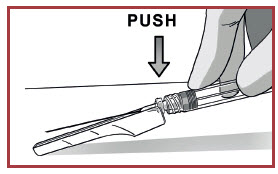
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Instruction for Use - 100mg
RISVAN®
(risperidone) extended-release injectable suspension
100 mg, single dose
For intramuscular injection
Do not administer by any other route
Must be reconstituted before use
IMPORTANT INFORMATION
- To be administered by a healthcare professional only.
- Label the administration syringe immediately after reconstitution, then administer immediately.
- To be administered intramuscularly only.
- Two administration safety needles are included: one for deltoid injection (21G, 1 inch) and the other for gluteus injection (20G, 2 inch).
- Do not substitute any component of the drug kit.
- As universal precaution, always wear gloves.
- The drug kit should be stored at room temperature between 20ºC – 25ºC (68ºF – 77ºF).
- Read the complete directions before use.
1. CHECK CONTENTS
Working on a clean surface, open the pouches and discard the desiccant.
Each carton of RISVAN ® contains:
- One pouch with a RISVAN ® prefilled syringe with a WHITE plunger rod and WHITE finger flange.
- One pouch with SOLVENT for RISVAN ® prefilled syringe with a TRANSPARENT plunger rod and a BLUE finger flange.
- Two administration needles 21G, 1 inch for deltoid (green cap) and a 20G, 2 inch for gluteus (yellow cap).
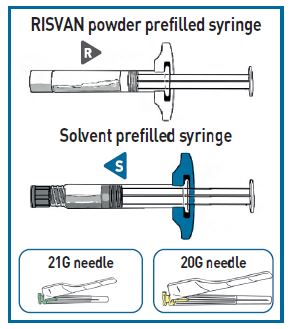
 Discard the kit if any component is damaged.
Discard the kit if any component is damaged.
Parenteral drug products should always be visually inspected for particles and discoloration prior to administration whenever the solution and container permit it.
1.1 Inspect solvent syringe
ENSURE that SOLVENT syringe content flows normally as a liquid. The solvent freezes at 66 ºF. If the solvent is frozen or partially frozen, warm it at room temperature until it flows normally.
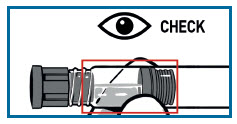
1.2. Dislodge powder syringe
TAP the RISVAN ® syringe to dislodge potential packed powder near the cap.
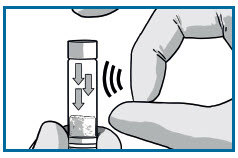
2. CONNECT THE SYRINGES
2.1. Uncap syringes in upright position
Hold both syringes in upright position to prevent loss of product. Figure A
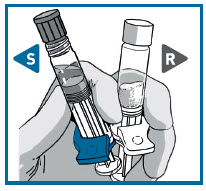
Figure A
PULL the cap off the Solvent syringe. Figure B
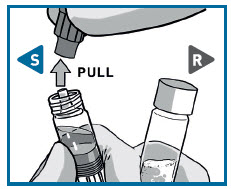
Figure B
TWIST and PULL the Powder syringe cap off. Figure C
 is in the upright position to prevent loss of product.
is in the upright position to prevent loss of product.
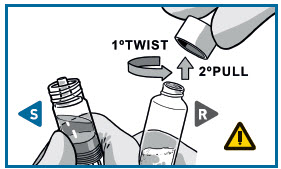
Figure C
2.2 Connect the syringes
Pick the solvent syringe or slightly tilt it vertically.
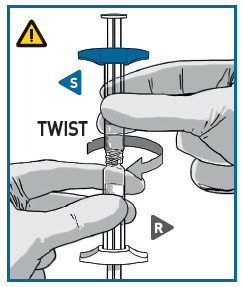
TWIST the syringes together until you feel a slight resistance.
 is in the upright position to prevent loss of product.
is in the upright position to prevent loss of product.
3. MIX THE CONTENTS
 STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
- PUSH VIGOROUSLY the Solvent content S towards the Powder syringe.
- DO NOT WAIT for powder wetting and QUICKLY start mixing contents by pushing the plungers FAST and alternately for 100 pushes (2 pushes within 1 second, approximately 1 minute).
- ENSUREmedication is passing between both syringes for proper mixing: medication is viscous and you will need to apply force when pressing on the plunger rods.
Mix for at least 100 pushes by doing alternately 1
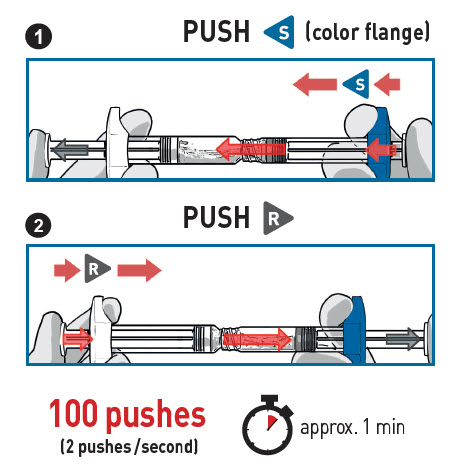
 Make sure medication is passing between both syringes
Make sure medication is passing between both syringes
When medication is correctly mixed, the appearance will be a uniform suspension off white to yellowish color andthick consistency.

 Proceed immediately to prepare the injection for administration
Proceed immediately to prepare the injection for administration
4. PREPARE INJECTION SYRINGE
4.1 Transfer medication
Place downward pressure on the R syringe that has the colored flange.
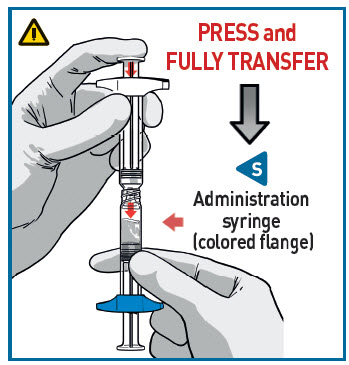
 Make sure all the content is transferred.
Make sure all the content is transferred.
4.2. Detach syringes
Once the medication is fully transferred, separate the two syringes by untwisting.
Proceed immediately to prepare the injection syringe for administration.
The injection must be given within 15 minutes after reconstitution.
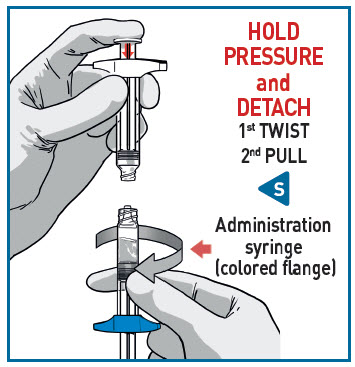
4.3. Attach the safety needle
Choose the proper needle:
- Deltoid: 21G, 1 inch for deltoid (green cap).
- Gluteus: 20G, 2 inch for gluteus (yellow cap).
Attach it using a clockwise twisting motion. Do not over-tighten.
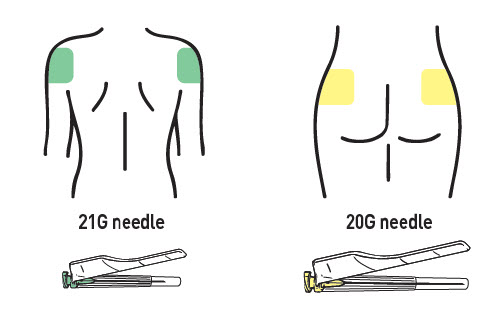
4.4 Remove excess air
Remove needle cover and push out the excess air (only big bubbles) from the syringe barrel.
 DO NOT expel any drops of medication
DO NOT expel any drops of medication
If you see the medication appearing at the needle tip, pull slightly back on the plunger to prevent medication spillage.
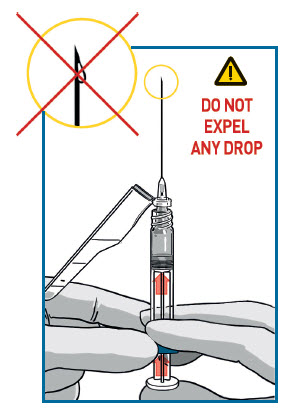
5. ADMINISTER AND DISPOSE
5.1 Inject medication
Insert the needle fully into the deltoid or gluteal muscle. DO NOT INJECT BY ANY OTHER ROUTE.
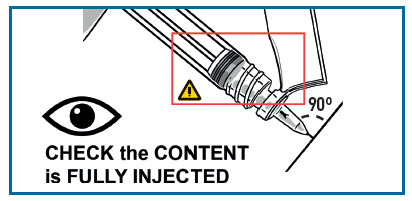
 WARNINGS
WARNINGS
- THICK MEDICATION, INJECT IT SLOWLY AND STEADILY. MAKE SURE TO FULLY INJECT IT.
- The injection time is longer than usual due to the viscosity of the medication.
- Wait a few seconds before removing the needle.
- Avoid inadvertent injection into a blood vessel.
5.2. Dispose of needle and syringe
Cover the needle by pressing on the needle guard using a finger or a flat surface and dispose immediately in a secure sharps disposal container.
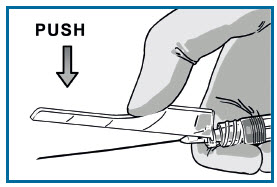
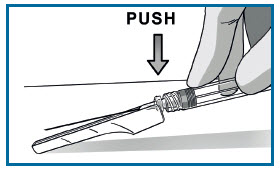
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Risvan 75 mg
Single-Dose Rx Only NDC: 82090-001-01
RISVAN ® (risperiDONE) 75 mg
For Extended-Release Injectable Suspension
FOR INTRAMUSCULAR INJECTION
Injection must be administered by a healthcare professional only. Administer once-monthly
Kit contents:
DO NOT SUBSTITUTE ANY COMPONENT OF THE KIT
- One pouch containing a prefilled syringe of RISVAN ® 75 mg marked with an R and a desicant.
- One pouch containing a prefilled syringe of SOLVENT marked with an S and a desiccant.
- Two sterile safety needles (One 21 gauge, 1 inch safety needle for administration in the deltoid muscle, and one 20 gauge, 2 inch safety needle for administration in the gluteus muscle).
Please read the Instructions for Use prior to administration
Must be reconstituted before use. Administer immediately after reconstitution

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Risvan 100 mg
Single-Dose Rx Only NDC: 82090-003-01
RISVAN ® (risperiDONE) 100 mg
For Extended-Release Injectable Suspension
FOR INTRAMUSCULAR INJECTION
Injection must be administered by a healthcare professional only. Administer once-monthly
Kit contents:
DO NOT SUBSTITUTE ANY COMPONENT OF THE KIT
- One pouch containing a prefilled syringe of RISVAN® 100 mg marked with an R and a desicant.
- One pouch containing a prefilled syringe of SOLVENT marked with an S and a desiccant.
- Two sterile safety needles (One 21 gauge, 1 inch safety needle for administration in the deltoid muscle, and one 20 gauge, 2 inch safety needle for administration in the gluteus muscle).
Please read the Instructions for Use prior to administration
Must be reconstituted before use. Administer immediately after reconstitution

| RISVAN
risperidone injection, powder, for suspension, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| RISVAN
risperidone injection, powder, for suspension, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Laboratorios Farmaceuticos Rovi, S.A. (462026238) |
Trademark Results [RISVAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RISVAN 79284764 not registered Live/Pending |
LABORATORIOS FARMACEUTICOS ROVI, S.A. 2020-04-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.