SIRTURO- bedaquiline fumarate tablet
SIRTURO by
Drug Labeling and Warnings
SIRTURO by is a Prescription medication manufactured, distributed, or labeled by Janssen Products, LP, Dishman Carbogen Amcis Limited, Janssen Pharmaceutica NV, Carbogen Amcis AG, Cilag AG, Recipharm Pharmaservices Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SIRTURO® safely and effectively. See full prescribing information for SIRTURO.
SIRTURO® (bedaquiline) tablets, for oral use
Initial U.S. Approval: 2012WARNING: INCREASED MORTALITY and QT PROLONGATION
See full prescribing information for complete boxed warning.
Increased Mortality
- An increased risk of death was seen in the SIRTURO treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial in adults. Only use SIRTURO in patients 12 years of age and older when an effective treatment regimen cannot otherwise be provided. (5.1)
QT Prolongation
- QT prolongation can occur with SIRTURO. Use with drugs that prolong the QT interval may cause additive QT prolongation. Monitor ECGs. Discontinue SIRTURO if significant ventricular arrhythmia or QTcF interval >500 ms develops. (5.2)
RECENT MAJOR CHANGES
Indications and Usage (1) 8/2019 Dosage and Administration, Recommended Dosage in Combination Therapy (2.3) 8/2019 Warnings and Precautions, Increased Mortality (5.1) 8/2019 Warnings and Precautions, Risk of Development of Resistance to Bedaquiline (5.3) 12/2019 Warnings and Precautions, Hepatotoxicity (5.4) 8/2019 INDICATIONS AND USAGE
SIRTURO is a diarylquinoline antimycobacterial drug indicated as part of combination therapy in adult and pediatric patients (12 to less than 18 years of age and weighing at least 30 kg) with pulmonary multi-drug resistant tuberculosis (MDR-TB). Reserve SIRTURO for use when an effective treatment regimen cannot otherwise be provided. (1)
This indication is approved under accelerated approval based on time to sputum culture conversion. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1, 14)
Limitations of Use: Do not use SIRTURO for the treatment of latent, extra-pulmonary or drug-sensitive tuberculosis or for the treatment of infections caused by non-tuberculous mycobacteria (1). Safety and efficacy of SIRTURO in HIV-infected patients with MDR-TB have not been established, as clinical data are limited. (14)
DOSAGE AND ADMINISTRATION
- Administer SIRTURO by directly observed therapy (DOT). (2.1)
- Emphasize need for compliance with full course of therapy. (2.1)
- Prior to administration, obtain ECG, liver enzymes and electrolytes. Obtain susceptibility information for the background regimen against Mycobacterium tuberculosis isolate if possible. (2.2)
- Only use SIRTURO in combination with at least 3 other drugs to which the patient's MDR-TB isolate has been shown to be susceptible in vitro. If in vitro testing results are unavailable, may initiate SIRTURO in combination with at least 4 other drugs to which patient's MDR-TB isolate is likely to be susceptible. (2.3)
- Recommended dosage in adult and pediatric patients (12 to less than 18 years of age and weighing at least 30 kg): 400 mg once daily for 2 weeks followed by 200 mg 3 times per week (with at least 48 hours between doses) for 22 weeks. (2.3)
- Swallow SIRTURO tablets whole with water and take with food. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 100 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- The most common adverse reactions reported in 10% or more of adult patients treated with SIRTURO were nausea, arthralgia, headache, hemoptysis and chest pain. (6.1)
- The most common adverse reactions reported in 10% or more of pediatric patients (12 to less than 18 years of age) treated with SIRTURO were arthralgia, nausea and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Therapeutics, Division of Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Lactation: Monitor infants exposed to bedaquiline through breast milk for signs of bedaquiline-related adverse reactions, such as hepatotoxicity. (6, 8.2)
- Pediatrics: The safety and effectiveness of SIRTURO in pediatric patients less than 12 years of age and/or weighing less than 30 kg have not been established. (8.4)
- Use with caution in patients with severe hepatic impairment and only when the benefits outweigh the risks. Monitor for SIRTURO-related adverse reactions. (8.6)
- Use with caution in patients with severe renal impairment. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY and QT PROLONGATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Required Testing Prior to Administration
2.3 Recommended Dosage in Combination Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality
5.2 QT Prolongation
5.3 Risk of Development of Resistance to Bedaquiline
5.4 Hepatotoxicity
5.5 Drug Interactions
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Inducers/Inhibitors
7.2 Other Antimicrobial Medications
7.3 Antiretroviral Medications
7.4 QT Interval Prolonging Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients (12 to less than 18 years of age)
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY and QT PROLONGATION
Increased Mortality
- An increased risk of death was seen in the SIRTURO treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial in adults. Only use SIRTURO in patients 12 years of age and older when an effective treatment regimen cannot otherwise be provided [see Indications and Usage (1), Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
QT Prolongation
- QT prolongation can occur with SIRTURO. Use with drugs that prolong the QT interval may cause additive QT prolongation. Monitor ECGs. Discontinue SIRTURO if significant ventricular arrhythmia or if QTcF interval prolongation of greater than 500 ms develops [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
SIRTURO is a diarylquinoline antimycobacterial drug indicated as part of combination therapy in the treatment of adult and pediatric patients (12 to less than 18 years of age and weighing at least 30 kg) with pulmonary multi-drug resistant tuberculosis (MDR-TB). Reserve SIRTURO for use when an effective treatment regimen cannot otherwise be provided.
This indication is approved under accelerated approval based on time to sputum culture conversion [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitations of Use:
- Do not use SIRTURO for the treatment of:
- Latent infection due to Mycobacterium tuberculosis
- Drug-sensitive tuberculosis
- Extra-pulmonary tuberculosis
- Infections caused by non-tuberculous mycobacteria
- The safety and efficacy of SIRTURO in the treatment of HIV infected patients with MDR-TB have not been established as clinical data are limited [see Clinical Studies (14)].
- Do not use SIRTURO for the treatment of:
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Administer SIRTURO by directly observed therapy (DOT).
- Use SIRTURO only in combination with other anti-mycobacterial drugs [see Dosage and Administration (2.3)].
- Emphasize the need for compliance with full course of therapy.
2.2 Required Testing Prior to Administration
Prior to treatment with SIRTURO, obtain the following:
- Susceptibility information for the background regimen against M. tuberculosis isolate if possible [see Dosage and Administration (2.3)]
- ECG [see Warnings and Precautions (5.2)]
- Serum potassium, calcium, and magnesium concentrations [see Warnings and Precautions (5.2)]
- Liver enzymes [see Warnings and Precautions (5.4)]
2.3 Recommended Dosage in Combination Therapy
Only use SIRTURO in combination with at least 3 other drugs to which the patient's MDR-TB isolate has been shown to be susceptible in vitro. If in vitro testing results are unavailable, SIRTURO treatment may be initiated in combination with at least 4 other drugs to which the patient's MDR-TB isolate is likely to be susceptible. Refer to the prescribing information of the drugs used in combination with SIRTURO.
See Table 1 for the recommended dosage of SIRTURO in adult and pediatric patients (12 to less than 18 years of age).
Table 1: Recommended Dosage of SIRTURO Population Dosage Adult patients (18 years of age and older) 400 mg orally once daily for the first two weeks, followed by 200 mg orally three times per week (with at least 48 hours between doses) for 22 weeks (total duration of 24 weeks) Pediatric patients (12 to less than 18 years of age) and weighing at least 30 kg The SIRTURO tablet should be swallowed whole with water and taken with food.
If a dose is missed during the first 2 weeks of treatment, do not administer the missed dose (skip the dose and then continue the daily dosing regimen). From Week 3 onwards, if a 200 mg dose is missed, administer the missed dose as soon as possible, and then resume the 3 times a week dosing regimen.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality
An increased risk of death was seen in the SIRTURO treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial in adults (based on the 120-week visit window). One death occurred during the 24 weeks of administration of SIRTURO. The imbalance in deaths is unexplained. No discernible pattern between death and sputum culture conversion, relapse, sensitivity to other drugs used to treat tuberculosis, HIV status, or severity of disease could be observed. Only use SIRTURO in patients 12 years of age and older when an effective treatment regimen cannot otherwise be provided [see Adverse Reactions (6)].
5.2 QT Prolongation
SIRTURO prolongs the QT interval. Obtain an ECG before initiation of treatment, and at least 2, 12, and 24 weeks after starting treatment with SIRTURO. Obtain serum potassium, calcium, and magnesium at baseline and correct if abnormal. Monitor electrolytes if QT prolongation is detected [see Adverse Reactions (6.1) and Drug Interactions (7.4)]. SIRTURO has not been studied in patients with ventricular arrhythmias or recent myocardial infarction.
The following may increase the risk for QT prolongation when patients are receiving SIRTURO:
- use with other QT prolonging drugs including fluoroquinolones and macrolide antibacterial drugs and the antimycobacterial drug, clofazimine
- a history of Torsade de Pointes
- a history of congenital long QT syndrome
- a history of or ongoing hypothyroidism
- a history of or ongoing bradyarrhythmias
- a history of uncompensated heart failure
- serum calcium, magnesium, or potassium levels below the lower limits of normal
If necessary, bedaquiline treatment initiation could be considered in these patients after a favorable benefit risk assessment and with frequent ECG monitoring.
Discontinue SIRTURO and all other QT prolonging drugs if the patient develops:
- Clinically significant ventricular arrhythmia
- A QTcF interval of greater than 500 ms (confirmed by repeat ECG)
If syncope occurs, obtain an ECG to detect QT prolongation.
5.3 Risk of Development of Resistance to Bedaquiline
A potential for development of resistance to bedaquiline in M. tuberculosis exists [see Microbiology (12.4)]. Bedaquiline must only be used in an appropriate combination regimen for the treatment of pulmonary MDR-TB to reduce the risk of development of resistance to bedaquiline [see Indications and Usage (1)].
5.4 Hepatotoxicity
In clinical trials, more hepatic-related adverse reactions were reported in adults with the use of SIRTURO plus other drugs used to treat tuberculosis compared to other drugs used to treat tuberculosis without the addition of SIRTURO. Alcohol and other hepatotoxic drugs should be avoided while on SIRTURO, especially in patients with impaired hepatic function. Hepatic-related adverse reactions have also been reported in pediatric patients 14 to less than 18 years of age [see Adverse Reactions (6.1)].
Monitor symptoms (such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness and hepatomegaly) and laboratory tests (ALT, AST, alkaline phosphatase, and bilirubin) at baseline, monthly while on treatment, and as needed. Test for viral hepatitis and discontinue other hepatotoxic medications if evidence of new or worsening liver dysfunction occurs. Discontinue SIRTURO if:
- aminotransferase elevations are accompanied by total bilirubin elevation greater than two times the upper limit of normal
- aminotransferase elevations are greater than eight times the upper limit of normal
- aminotransferase elevations are greater than five times the upper limit of normal and persist beyond two weeks
5.5 Drug Interactions
CYP3A4 inducers/inhibitors
Bedaquiline is metabolized by CYP3A4 and its systemic exposure and therapeutic effect may therefore be reduced during co-administration with inducers of CYP3A4. Avoid co-administration of strong CYP3A4 inducers, such as rifamycins (i.e., rifampin, rifapentine and rifabutin), or moderate CYP3A4 inducers, such as efavirenz, during treatment with SIRTURO [see Drug Interactions (7.1)].
Co-administration of SIRTURO with strong CYP3A4 inhibitors may increase the systemic exposure to bedaquiline, which could potentially increase the risk of adverse reactions. Therefore, avoid the use of strong CYP3A4 inhibitors for more than 14 consecutive days while on SIRTURO, unless the benefit of treatment with the drug combination outweighs the risk [see Drug Interactions (7.1)]. Appropriate clinical monitoring for SIRTURO-related adverse reactions is recommended.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Increased mortality [see Warnings and Precautions (5.1)]
- QT Prolongation [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Drug Interactions [see Warnings and Precautions (5.5)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Use SIRTURO only in combination with other anti-mycobacterial drugs [see Dosage and Administration (2.3)]. Refer to the prescribing information of the drugs used in combination with SIRTURO for their respective adverse reactions.
Clinical Studies Experience in Adults
Adverse drug reactions for SIRTURO were identified from the pooled safety data from 335 SIRTURO-exposed patients who received 8 weeks (Study 2) and 24 weeks (Studies 1 and 3) at the proposed dose. Studies 1 and 2 were randomized, double-blind, placebo-controlled trials in newly diagnosed patients with pulmonary MDR-TB. In both treatment arms, patients received SIRTURO or placebo in combination with other drugs used to treat MDR-TB. Study 3 was an open-label, noncomparative study with SIRTURO administered as part of an individualized pulmonary MDR-TB treatment regimen in previously treated patients.
In Study 1, 35% were Black, 17.5% were Hispanic, 12.5% were White, 9.4% were Asian, and 25.6% were of another race. Eight of 79 (10.1%) patients in the SIRTURO group and 16 of 81 (19.8%) patients in the placebo treatment group were HIV-infected. Seven (8.9%) SIRTURO-treated patients and six (7.4%) placebo-treated patients discontinued Study 1 because of an adverse reaction.
Table 2: Select Adverse Reactions from Study 1 That Occurred More Frequently Than Placebo During Treatment with SIRTURO Adverse Reactions SIRTURO Treatment Group
N=79
n (%)Placebo Treatment Group
N=81
n (%)- * Terms represented by 'transaminases increased' included transaminases increased, AST increased, ALT increased, hepatic enzyme increased, and hepatic function abnormal.
Nausea 30 (38) 26 (32) Arthralgia 26 (33) 18 (22) Headache 22 (28) 10 (12) Hemoptysis 14 (18) 9 (11) Chest Pain 9 (11) 6 (7) Anorexia 7 (9) 3 (4) Transaminases Increased* 7 (9) 1 (1) Rash 6 (8) 3 (4) Blood Amylase Increased 2 (3) 1 (1) No additional unique adverse reactions were identified from the uncontrolled Study 3.
In both Studies 1 and 2, aminotransferase elevations of at least 3 times the upper limit of normal developed more frequently in the SIRTURO treatment group (11/102 [10.8%] vs 6/105 [5.7%]) than in the placebo treatment group. In Study 3, 22/230 (9.6%) patients had alanine aminotransferase or aspartate aminotransferase greater than or equal to 3 times the upper limit of normal during the overall treatment period.
Increased Mortality
In Study 1, there was a statistically significant increased mortality risk by Week 120 in the SIRTURO treatment group compared to the placebo treatment group (9/79 (11.4%) versus 2/81 (2.5%), p-value=0.03, an exact 95% confidence interval of the difference [1.1%, 18.2%]). Five of the 9 SIRTURO deaths and the 2 placebo deaths were tuberculosis-related. One death occurred during the 24-week SIRTURO treatment period. The median time to death for the remaining eight patients in the SIRTURO treatment group was 329 days after last intake of SIRTURO. The imbalance in deaths is unexplained; no discernible pattern between death and sputum conversion, relapse, sensitivity to other drugs used to treat tuberculosis, HIV status, and severity of disease was observed.
In the open-label Study 3, 6.9% (16/233) of patients died. The most common cause of death as reported by the investigator was TB (9 patients). All but one patient who died of TB had not converted or had relapsed. The causes of death in the remaining patients varied.
Clinical Studies Experience in Pediatric Patients
The safety assessment of bedaquiline is based on the Week 24 analysis of the single-arm, open-label trial, TMC207-C211, in 15 pediatric patients. The trial was designed to enroll patients 12 to less than 18 years of age (only patients 14 to less than 18 years of age were enrolled) with confirmed or probable pulmonary MDR-TB infection who received SIRTURO at the recommended dosage regimen in combination with a background regimen [see Clinical Studies (14.2)].
The most common adverse drug reactions were arthralgia in 6/15 (40%) patients, nausea in 2/15 (13%) patients, and abdominal pain in 2/15 (13%) patients. Among the 15 patients, no deaths occurred during treatment with SIRTURO. Observed laboratory abnormalities were comparable to those in adults.
-
7 DRUG INTERACTIONS
7.1 CYP3A4 Inducers/Inhibitors
Bedaquiline exposure may be reduced during co-administration with inducers of CYP3A4 and increased during co-administration with inhibitors of CYP3A4.
CYP3A4 Inducers
Due to the possibility of a reduction of the therapeutic effect of bedaquiline because of the decrease in systemic exposure, co-administration of strong CYP3A4 inducers, such as rifamycins (i.e., rifampin, rifapentine and rifabutin), or moderate CYP3A4 inducers should be avoided during treatment with SIRTURO [see Clinical Pharmacology (12.3)].
CYP3A4 inhibitors
Due to the potential risk of adverse reactions to bedaquiline because of the increase in systemic exposure, prolonged co-administration of bedaquiline and strong CYP3A4 inhibitors, such as ketoconazole or itraconazole, for more than 14 consecutive days should be avoided unless the benefit outweighs the risk [see Clinical Pharmacology (12.3)]. Appropriate clinical monitoring for SIRTURO-related adverse reactions is recommended.
7.2 Other Antimicrobial Medications
No dose-adjustment of isoniazid or pyrazinamide is required during co-administration with SIRTURO.
In a placebo-controlled clinical trial in adult patients with MDR-TB, no major impact of co-administration of SIRTURO on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
7.3 Antiretroviral Medications
Lopinavir/ritonavir
Although clinical data in HIV/MDR-TB co-infected patients on the combined use of lopinavir (400 mg)/ritonavir (100 mg) with SIRTURO are not available, use SIRTURO with caution when co-administered with lopinavir/ritonavir and only if the benefit outweighs the risk [see Clinical Pharmacology (12.3)].
Nevirapine
No dosage adjustment of bedaquiline is required when co-administered with nevirapine [see Clinical Pharmacology (12.3)].
Efavirenz
Concomitant administration of bedaquiline and efavirenz, or other moderate CYP3A inducers, should be avoided [see Warnings and Precautions (5.5)].
7.4 QT Interval Prolonging Drugs
In a drug interaction study of bedaquiline and ketoconazole in adults, a greater effect on QTc was observed after repeated dosing with bedaquiline and ketoconazole in combination than after repeated dosing with the individual drugs. Additive or synergistic QT prolongation was observed when bedaquiline was co-administered with other drugs that prolong the QT interval.
In Study 3, mean increases in QTc were larger in the 17 adult patients who were taking clofazimine with bedaquiline at Week 24 (mean change from reference of 31.9 ms) than in patients who were not taking clofazimine with bedaquiline at Week 24 (mean change from baseline of 12.3 ms). Monitor ECGs if SIRTURO is co-administered to patients receiving other drugs that prolong the QTc interval, and discontinue SIRTURO if evidence of serious ventricular arrhythmia or QTcF interval greater than 500 ms. [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature of SIRTURO use in pregnant women are insufficient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks associated with active tuberculosis during pregnancy (see Clinical Considerations).
Reproduction studies performed in rats and rabbits have revealed no evidence of harm to the fetus due to oral administration of bedaquiline to pregnant rats and rabbits during organogenesis at exposures up to 6 times the clinical dose based on AUC comparisons (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Pregnant rats were treated with bedaquiline at 5, 15 and 45 mg/kg (approximately 0.7, 2 and 6 times the clinical dose based on AUC comparisons) during the period of organogenesis (gestational Days 6–17, inclusive). Pregnant rabbits were treated with bedaquiline at 10, 30 and 100 mg/kg (approximately 0.05, 0.2 and 1.5 times the clinical dose based on AUC comparisons) during the period of organogenesis (gestational Days 6–19, inclusive). No embryotoxic effects were found in rats or rabbits at dose exposures up to 6 times the clinical dose exposures based on AUC comparisons.
8.2 Lactation
Risk Summary
There is no information regarding the presence of bedaquiline in human milk. Minimal data are available on the effects of the drug on breastfed infants. No data are available on the effects of the drug on milk production. Bedaquiline is concentrated in the milk of rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SIRTURO and any potential adverse effects on the breastfed infant from SIRTURO or from the underlying maternal condition.
Clinical Considerations
Monitor infants exposed to bedaquiline through breast milk for signs of bedaquiline-related adverse reactions, such as hepatotoxicity [see Adverse Reactions (6)].
Data
Bedaquiline concentrations in rat milk were 6-fold to 12-fold higher than the maximum concentration observed in maternal plasma at exposures 1 time to 2 times the clinical exposure (based on AUC comparisons). Pups from these dams were exposed to bedaquiline via milk during the lactation period and showed reduced body weights compared to control animals.
8.4 Pediatric Use
The safety and effectiveness of SIRTURO have been established in pediatric patients 12 to less than 18 years of age and weighing at least 30 kg. The use of SIRTURO in this pediatric population is supported by evidence from the study of SIRTURO in adults together with additional pharmacokinetic and safety data from the single-arm, open-label, trial that enrolled 15 pediatric patients 14 to less than 18 years of age with confirmed or probable MDR-TB infection who were treated with SIRTURO for 24 weeks in combination with a background regimen [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. The use of SIRTURO in pediatric patients 12 to less than 14 years of age is based on information obtained from the studies conducted in adults and pediatric patients 14 to less than 18 years of age [see Adverse Reactions (6.1) and Clinical Studies (14.2)].
The safety and effectiveness of SIRTURO in pediatric patients less than 12 years of age and/or weighing less than 30 kg have not been established.
8.5 Geriatric Use
Because of limited data, differences in outcomes or specific risks with SIRTURO cannot be ruled out for patients 65 years of age and older.
8.6 Hepatic Impairment
The pharmacokinetics of bedaquiline were assessed after single-dose administration to adult patients with moderate hepatic impairment (Child-Pugh B) [see Clinical Pharmacology (12.3)]. Based on these results, no dose adjustment is necessary for SIRTURO in patients with mild or moderate hepatic impairment. SIRTURO has not been studied in patients with severe hepatic impairment and should be used with caution in these patients only when the benefits outweigh the risks. Clinical monitoring for SIRTURO-related adverse reactions is recommended [see Warnings and Precautions (5.4)].
8.7 Renal Impairment
SIRTURO has mainly been studied in adult patients with normal renal function. Renal excretion of unchanged bedaquiline is not substantial (less than or equal to 0.001%). No dose adjustment is required in patients with mild or moderate renal impairment. In patients with severe renal impairment or end stage renal disease requiring hemodialysis or peritoneal dialysis, SIRTURO should be used with caution [see Clinical Pharmacology (12.3)]. Monitor adult and pediatric patients for adverse reactions of SIRTURO when administered to patients with severe renal impairment or end stage renal disease requiring hemodialysis or peritoneal dialysis.
-
10 OVERDOSAGE
There is no experience with the treatment of acute overdose with SIRTURO. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in case of deliberate or accidental overdose. It is advisable to contact a poison control center to obtain the latest recommendations for the management of an overdose. Since bedaquiline is highly protein-bound, dialysis is not likely to significantly remove bedaquiline from plasma.
-
11 DESCRIPTION
SIRTURO (bedaquiline) is a diarylquinoline antimycobacterial drug containing bedaquiline as the fumarate salt. SIRTURO is available as 100 mg strength tablets for oral administration. Each tablet contains 100 mg of bedaquiline (equivalent to 120.89 mg of bedaquiline fumarate).
Bedaquiline fumarate is a white to almost white powder and is practically insoluble in aqueous media. The chemical name of bedaquiline fumarate is (1R, 2S)-1-(6-bromo-2-methoxy-3-quinolinyl)-4-(dimethylamino)-2-(1-naphthalenyl)-1-phenyl-2-butanol compound with fumaric acid (1:1). It has a molecular formula of C32H31BrN2O2∙C4H4O4 and a molecular weight of 671.58 (555.50 + 116.07). The molecular structure of bedaquiline fumarate is the following:
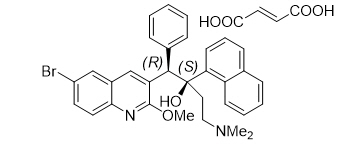
SIRTURO (bedaquiline) contains the following inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose 2910 15 mPa.s, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, purified water (removed during processing).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bedaquiline is a diarylquinoline antimycobacterial drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
Bedaquiline is primarily subjected to oxidative metabolism leading to the formation of N-monodesmethyl metabolite (M2). M2 is not thought to contribute significantly to clinical efficacy given its lower average exposure (23% to 31%) in humans and lower antimycobacterial activity (4-fold to 6-fold lower) compared to the parent compound. However, M2 plasma concentrations appeared to correlate with QT prolongation.
Cardiac Electrophysiology
In Study 1, in adults, the mean increases in QTcF, corrected using the Fridericia method, were greater in the SIRTURO treatment group compared to the placebo treatment group from the first week of treatment (9.9 ms at Week 1 for SIRTURO and 3.5 ms for placebo). The largest mean increase in QTcF during the 24 weeks of SIRTURO treatment was 15.7 ms compared to 6.2 ms with placebo treatment (at Week 18). After bedaquiline treatment ended, the QTcF gradually decreased, and the mean value was similar to that in the placebo group by study week 60.
In Study 3, where adult patients with no treatment options received other QT-prolonging drugs used to treat tuberculosis, including clofazimine, concurrent use with SIRTURO resulted in additive QTcF prolongation, proportional to the number of QT prolonging drugs in the treatment regimen. Patients taking SIRTURO alone with no other QT prolonging drug developed a mean QTcF increase over baseline of 23.7 ms with no QTcF segment duration in excess of 480 ms, whereas patients taking at least 2 other QT prolonging drugs developed a mean QTcF prolongation of 30.7 ms over baseline, and resulted in QTcF segment duration in excess of 500 ms in one patient [see Warnings and Precautions (5.2)].
12.3 Pharmacokinetics
The pharmacokinetic parameters of bedaquiline in adult MDR-TB patients at the recommended dosing regimen of SIRTURO (400 mg for 2 weeks followed by 200 mg three times per week for 22 weeks) in combination with a background regimen are provided in Table 3.
Table 3: Pharmacokinetic Parameters of Bedaquiline Following Repeat Dose Administration of SIRTURO at the Recommended Dosing Regimen to Adult MDR-TB Patients at Week 8 Administered with Food (N = 18) Pharmacokinetic Parameter Bedaquiline
Mean (SD)SD=Standard deviation - * Median (range)
AUC24h (ng∙h/mL) 25,863 (13,259) Cmax (ng/mL) 1,659 (722) Tmax (h)* 5 (3–8) Cmin (ng/mL) 654 (498) Absorption
After single oral dose administration of SIRTURO, maximum plasma concentrations (Cmax) are typically achieved at approximately 5 hours post-dose. Cmax and the area under the plasma concentration-time curve (AUC) increased proportionally up 700 mg (1.75 times the 400 mg loading dose).
Administration of SIRTURO with a standard meal containing approximately 22 grams of fat (558 total Kcal) increased the relative bioavailability by approximately 2-fold compared to administration under fasted conditions. SIRTURO should be taken with food to enhance its oral bioavailability.
Distribution
The plasma protein binding of bedaquiline is greater than 99.9%. The volume of distribution in the central compartment is estimated to be approximately 164 Liters.
Elimination
After reaching Cmax, bedaquiline concentrations decline tri-exponentially. The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. This long terminal elimination phase likely reflects slow release of bedaquiline and M2 from peripheral tissues.
Specific Populations
Hepatic Impairment: After single-dose administration of 400 mg SIRTURO to 8 adult patients with moderate hepatic impairment (Child-Pugh B), mean exposure to bedaquiline and M2 (AUC672h) was approximately 20% lower compared to healthy subjects. SIRTURO has not been studied in patients with severe hepatic impairment. [See Warnings and Precautions (5.4) and Use in Specific Populations (8.6)].
Renal Impairment: SIRTURO has mainly been studied in adult patients with normal renal function. Renal excretion of unchanged bedaquiline is not substantial (less than or equal to 0.001%).
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO 200 mg three times per week, creatinine clearance was not found to influence the pharmacokinetic parameters of bedaquiline. It is therefore not expected that mild or moderate renal impairment will have a clinically relevant effect on the exposure to bedaquiline. However, in patients with severe renal impairment or end-stage renal disease requiring hemodialysis or peritoneal dialysis bedaquiline concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction. As bedaquiline is highly bound to plasma proteins, it is unlikely that it will be significantly removed from plasma by hemodialysis or peritoneal dialysis [see Use in Specific Populations (8.7)].
Sex: In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO no clinically relevant difference in exposure between men and women were observed.
Race/Ethnicity: In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO, systemic exposure (AUC) to bedaquiline was found to be 34% lower in Black patients than in patients from other race categories. This lower exposure was not considered to be clinically relevant as no clear relationship between exposure to bedaquiline and response has been observed in clinical trials of MDR-TB. Furthermore, response rates were comparable in patients of different race categories that completed 24 weeks of bedaquiline treatment.
HIV Co-infection: There are limited data on the use of SIRTURO in HIV co-infected patients [see Drug Interactions (7)].
Geriatric Population: There are limited data on the use of SIRTURO in tuberculosis patients 65 years of age and older.
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO, age was not found to influence the pharmacokinetics of bedaquiline.
Pediatric Population: The pharmacokinetic parameters of bedaquiline in 15 MDR-TB pediatric patients 14 to less than 18 years of age who received the same adult dosage regimen of SIRTURO (400 mg once daily for the first 2 weeks and 200 mg 3 times/week for the following 22 weeks) in combination with a background regimen were comparable to those in adults; see Table 4 below for a summary of the pharmacokinetic parameters. There was no impact of body weight on bedaquiline pharmacokinetics in pediatric MDR-TB patients 14 to less than 18 years of age (38 to 75 kg).
Table 4: Pharmacokinetic Parameters of Bedaquiline Following Repeat Dose Administration of SIRTURO to Pediatric MDR-TB Patients 14 to less than 18 years of age at Week 12 Administered with Food (N=15) Pharmacokinetic Parameter Bedaquiline
Mean (SD)SD=Standard Deviation - * Median (range)
AUC24h (ng∙h/mL) 26,300 (10,300) Cmax (ng/mL) 1,800 (736) Tmax (h)* 4 (2–8) Cmin (ng/mL) 544 (263) The pharmacokinetics of SIRTURO in pediatric patients less than 14 years of age or weighing less than 38 kg have not been evaluated.
Drug-Drug Interactions
In vitro, bedaquiline does not significantly inhibit the activity of the following CYP450 enzymes that were tested: CYP1A2, CYP2A6, CYP2C8/9/10, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A4/5 and CYP4A, and it does not induce CYP1A2, CYP2C9, CYP2C19, or CYP3A4 activities.
Bedaquiline is an in vitro substrate of CYP3A4, and because of this, the following clinical drug interaction studies were performed.
Ketoconazole: Co-administration of multiple-dose bedaquiline (400 mg once daily for 14 days) and multiple-dose ketoconazole (once daily 400 mg for 4 days) in healthy adult subjects increased the AUC24h, Cmax and Cmin of bedaquiline by 22% [90% CI (12; 32)], 9% [90% CI (-2, 21)] and 33% [90% CI (24, 43)] respectively [see Drug Interactions (7.1) and (7.4)].
Rifampin: In a drug interaction study of single-dose 300 mg bedaquiline and multiple-dose rifampin (once daily 600 mg for 21 days) in healthy adult subjects, the exposure (AUC) to bedaquiline was reduced by 52% [90% CI (-57; -46)] [see Drug Interactions (7.1)].
Antimicrobial agents: The combination of multiple-dose bedaquiline 400 mg once daily with multiple-dose isoniazid/pyrazinamide (300 mg/2000 mg once daily) in healthy adult subjects did not result in clinically relevant changes in the exposure (AUC) to bedaquiline, isoniazid or pyrazinamide [see Drug Interactions (7.2)].
In a placebo-controlled study in adult patients with MDR-TB, no major impact of co-administration of bedaquiline on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
Lopinavir/ritonavir: In a drug interaction study in healthy adult volunteers of single-dose bedaquiline (400 mg) and multiple-dose lopinavir (400 mg)/ritonavir (100 mg) given twice daily for 24 days, the mean AUC of bedaquiline was increased by 22% [90% CI (11; 34)] while the mean Cmax was not substantially affected [see Drug Interactions (7.3)].
Nevirapine: Co-administration of multiple-dose nevirapine 200 mg twice daily for 4 weeks in HIV-infected adult patients with a single 400 mg dose of bedaquiline did not result in clinically relevant changes in the exposure to bedaquiline [see Drug Interactions (7.3)].
Efavirenz: Co-administration of a single dose of bedaquiline 400 mg and efavirenz 600 mg daily for 27 days to healthy adult volunteers resulted in approximately a 20% decrease in the AUCinf of bedaquiline; the Cmax of bedaquiline was not altered. The AUC and Cmax of the primary metabolite of bedaquiline (M2) were increased by 70% and 80%, respectively. The effect of efavirenz on the pharmacokinetics of bedaquiline and M2 following steady-state administration of bedaquiline has not been evaluated [see Drug Interactions (7.3)].
12.4 Microbiology
Mechanism of Action
SIRTURO is a diarylquinoline antimycobacterial drug that inhibits mycobacterial ATP (adenosine 5'-triphosphate) synthase, by binding to subunit c of the enzyme that is essential for the generation of energy in M. tuberculosis.
Resistance
A potential for development of resistance to bedaquiline in M. tuberculosis exists. Modification of the atpE target gene, and/or upregulation of the MmpS5-MmpL5 efflux pump (Rv0678 mutations) have been associated with increased bedaquiline MIC values in isolates of M. tuberculosis. Target-based mutations generated in preclinical studies lead to 8- to 133-fold increases in bedaquiline MIC, resulting in MICs ranging from 0.25 to 4 micrograms per mL. Efflux-based mutations have been seen in preclinical and clinical isolates. These lead to 2- to 8-fold increases in bedaquiline MICs, resulting in bedaquiline MICs ranging from 0.25 to 0.5 micrograms per mL.
M. tuberculosis isolates from a clinical study in adult patients with MDR-TB that developed at least 4-fold increase in bedaquiline MIC were associated with mutations in Rv0678 gene that lead to upregulation of the MmpS5-MmpL5 efflux pump. Isolates with these efflux-based mutations are less susceptible to clofazimine. Isolates that are phenotypically resistant to bedaquiline should be tested for cross-resistance to clofazimine, if clofazimine is being considered as part of the treatment regimen. In the Phase II trials there was no clear relationship between the presence of Rv0678 mutations at baseline and treatment outcome.
Activity In Vitro and in Clinical Infections
SIRTURO has been shown to be active in vitro and in clinical infections against most isolates of M. tuberculosis [see Indications and Usage (1) and Clinical Studies (14)].
Susceptibility Testing
The bedaquiline agar (left) and resazurin microtiter assay1 (REMA; a 7H9 broth microdilution to which resazurin, a bacterial growth indicator, was added) (right) MIC distributions against clinical isolates resistant to isoniazid and rifampin from Studies 1, 2, and 3 are provided below.
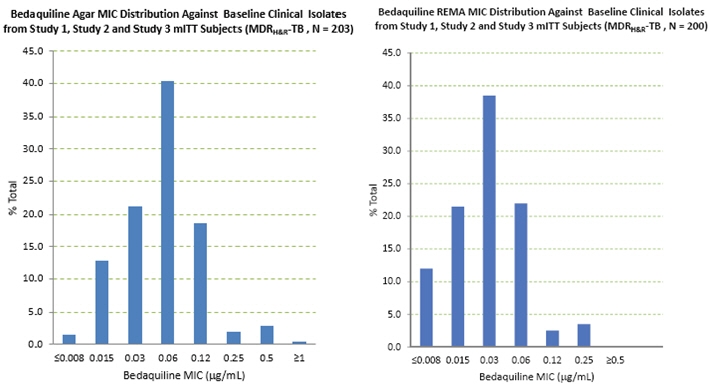
Figure 1: Bedaquiline MIC Distribution against Baseline MDRH&R-TB Isolates from Studies 1, 2, and 3 mITT Adult Patients: Agar Method (left) and Broth (REMA) Method (right) MICs for baseline M. tuberculosis isolates from patients in Studies 1 and 3 and their sputum culture conversion rates at Week 24 are shown in Table 5 below. Based on the available data, there was no trend for poor microbiologic outcomes related to baseline bedaquiline MIC.
Table 5: Culture Conversion Rates (Week 24 Data Selection, No Overruling for Discontinuation) at Week 24 By Baseline Bedaquiline MIC for mITT Patients from Study 1 and Study 3 Baseline Bedaquiline MIC
(micrograms/mL)SIRTURO (Bedaquiline) Treatment Group
24-Week Culture Conversion Rate
n/N (%)7H11 Agar 7H9 Broth (REMA) N=number of patients with data; n=number of patients with that result; MIC=minimum inhibitory concentration; BR=background regimen ≤ 0.008 2/2 (100) 21/25 (84.0) 0.015 13/15 (86.7) 33/39 (84.6) 0.03 36/46 (78.3) 70/92 (76.1) 0.06 82/107 (76.6) 45/56 (80.4) 0.12 36/42 (85.7) 6/7 (85.7) 0.25 3/4 (75.0) 3/4 (75.0) 0.5 5/6 (83.3) 0/1 (0) ≥ 1 0/1 (0) Nineteen patients in the efficacy population of study 3 had bedaquiline susceptibility testing results of paired (baseline and post-baseline, all of which were at Week 24 or later) genotypically identical isolates. Twelve of the 19 had a post-baseline ≥4-fold increase in bedaquiline MIC. Whole genome sequencing of 9 of these 12 post-baseline isolates was done and no mutations were found in the ATP synthase operon. All 9 were found to have a mutation in Rv0678. Eleven of the twelve (11/12) increases in bedaquiline MIC were seen in patients with pre-XDR-TB or with XDR-TB. Pre-XDR-TB is defined as MDR-TB isolates resistant to either a fluoroquinolone or a second line injectable drug, and XDR-TB as MDR-TB isolates resistant to both a fluoroquinolone and a second line injectable drug. Based on available data, response rate (culture conversion at week 120 endpoint) was similar in patients with ≥4-fold increases in bedaquiline MIC (5/12) and patients with < 4-fold increases (3/7).
For specific information regarding susceptibility test criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: www.fda.gov/STIC.
-
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Bedaquiline was not carcinogenic in rats up to the maximum tolerated dose of 10 mg/kg/day. Exposures at this dose in rats (AUCs) were within 1-fold to 2-fold of those observed in adult patients in the clinical trials.
No mutagenic or clastogenic effects were detected in the in vitro non-mammalian reverse mutation (Ames) test, in vitro mammalian (mouse lymphoma) forward mutation assay and an in vivo mouse bone marrow micronucleus assay.
SIRTURO did not affect fertility when evaluated in male and female rats at approximately twice the clinical exposure based on AUC comparisons. There was no effect of maternal treatment on sexual maturation, mating performance or fertility in F1 generation exposed to bedaquiline in utero at approximately twice the human exposure.
13.2 Animal Toxicology and/or Pharmacology
Bedaquiline is a cationic, amphiphilic drug that induced phospholipidosis (at almost all doses, even after very short exposures) in drug-treated animals, mainly in cells of the monocytic phagocytic system (MPS). All species tested showed drug-related increases in pigment-laden and/or foamy macrophages, mostly in the lymph nodes, spleen, lungs, liver, stomach, skeletal muscle, pancreas and/or uterus. After treatment ended, these findings were slowly reversible. Muscle degeneration was observed in several species at the highest doses tested. For example, the diaphragm, esophagus, quadriceps and tongue of rats were affected after 26 weeks of treatment at doses similar to clinical exposures based on AUC comparisons. These findings were not seen after a 12-week, treatment-free, recovery period and were not present in rats given the same dose biweekly. Degeneration of the fundic mucosa of the stomach, hepatocellular hypertrophy and pancreatitis were also seen.
-
14 CLINICAL STUDIES
14.1 Adult Patients
A placebo-controlled, double-blind, randomized trial (Study 1) was conducted in patients with newly diagnosed sputum smear-positive MDR pulmonary M. tuberculosis. All patients received a combination of five other antimycobacterial drugs used to treat MDR-TB (i.e., ethionamide, kanamycin, pyrazinamide, ofloxacin, and cycloserine/terizidone or available alternative) for a total duration of 18–24 months or at least 12 months after the first confirmed negative culture. In addition to this regimen, patients were randomized to receive 24 weeks of treatment with SIRTURO 400 mg once daily for the first 2 weeks followed by 200 mg 3 times per week for 22 weeks or matching placebo for the same duration. Overall, 79 patients were randomized to the SIRTURO arm and 81 to the placebo arm. A final evaluation was conducted at Week 120.
Sixty-seven patients randomized to SIRTURO and 66 patients randomized to placebo had confirmed MDR-TB, based on susceptibility tests (taken prior to randomization) or medical history if no susceptibility results were available, and were included in the efficacy analyses. Demographics were as follows: 63% of the study population was male, with a median age of 34 years, 35% were Black, and 15% were HIV-positive (median CD4 cell count 468 cells/µL). Most patients had cavitation in one lung (62%); and 18% of patients had cavitation in both lungs.
Time to sputum culture conversion was defined as the interval in days between the first dose of study drug and the date of the first of two consecutive negative sputum cultures collected at least 25 days apart during treatment. In this trial, the SIRTURO treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 24. Median time to culture conversion was 83 days for the SIRTURO treatment group compared to 125 days for the placebo treatment group. Table 6 shows the proportion of patients with sputum culture conversion at Week 24 and Week 120.
Table 6: Culture Conversion Status in Patients with MDR-TB at Week 24 and Week 120 in Study 1 Microbiologic Status SIRTURO (24 weeks) + combination of other antimycobacterial drugs
N=67Placebo (24 weeks) + combination of other antimycobacterial drugs
N=66Difference [95% CI]
p-value- * A patient's reason for treatment failure was counted only in the first row for which a patient qualifies.
- † Patients received 24 weeks of SIRTURO or placebo for the first 24 weeks and received a combination of other antimycobacterial drugs for up to 96 weeks.
Week 24 Sputum Culture Conversion 78% 58% 20.0% [4.5%, 35.6%]
0.014Treatment failure* 22% 42% Died 1% 0% Lack of conversion 21% 35% Discontinuation 0% 8% Week 120† Sputum Culture Conversion 61% 44% 17.3% [0.5%, 34.0%]
0.046Treatment failure* 39% 56% Died 12% 3% Lack of conversion/relapse 16% 35% Discontinuation 10% 18% Study 2 was a smaller placebo-controlled study designed similarly to Study 1 except that SIRTURO or placebo was given for only 8 weeks instead of 24 weeks. Patients were randomized to either SIRTURO and other drugs used to treat MDR-TB (SIRTURO treatment group) (n=23) or placebo and other drugs used to treat MDR-TB (placebo treatment group) (n=24). Twenty-one patients randomized to the SIRTURO treatment group and 23 patients randomized to the placebo treatment group had confirmed MDR-TB based on patients' baseline M. tuberculosis isolate obtained prior to randomization. The SIRTURO treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 8. At Weeks 8 and 24, the differences in culture conversion proportions were 38.9% (95% CI: [12.3%, 63.1%] and p-value: 0.004), 15.7% (95% CI: [-11.9%, 41.9%] and p-value: 0.32), respectively.
Study 3 was a Phase 2b, uncontrolled study to evaluate the safety, tolerability, and efficacy of SIRTURO as part of an individualized MDR-TB treatment regimen in 233 patients with sputum smear positive (within 6 months prior to screening) pulmonary MDR-TB. Patients received SIRTURO for 24 weeks in combination with antibacterial drugs. Upon completion of the 24-week treatment with SIRTURO, all patients continued to receive their background regimen in accordance with national TB program (NTP) treatment guidelines. A final evaluation was conducted at Week 120. Treatment responses to SIRTURO at week 120 were generally consistent with those from Study 1.
14.2 Pediatric Patients (12 to less than 18 years of age)
The pediatric trial, TMC207-C211(NCT02354014), was designed as a single-arm, open-label, trial to evaluate the pharmacokinetics, safety and tolerability of SIRTURO in combination with a background regimen in patients 12 to less than 18 years of age with confirmed or probable pulmonary MDR-TB infection. Fifteen patients ages 14 to less than 18 years of age were enrolled in the study. The median age was 16 years, 80% were female, 53% were Black, 33% were White and 13% were Asian. No patient 12 to less than 14 years of age was enrolled in the study. SIRTURO was administered as 400 mg once daily for the first 2 weeks and 200 mg 3 times/week for the following 22 weeks.
In the subset of patients with culture positive pulmonary MDR-TB at baseline, treatment with bedaquiline resulted in conversion to a negative culture in 75.0% (6/8 patients) at Week 24.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SIRTURO is supplied as uncoated white to almost white round biconvex 100 mg tablets with debossing of "T" over "207" on one side and "100" on the other side. The tablets are packaged in white high density polyethylene (HDPE) bottles with child-resistant polypropylene (PP) closure with induction seal liner. Each bottle contains 188 tablets.
NDC: 59676-701-01
16.2 Storage and Handling
Dispense in original container. Store tablets dispensed outside the original container in a tight light-resistant container with an expiration date not to exceed 3 months.
Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Adverse Reactions
Advise patients that the following serious side effects can occur with SIRTURO: death, heart rhythm abnormalities, and/or hepatitis. In addition, advise patients about other potential side effects: nausea, joint pain, headache, increased blood amylase, hemoptysis, chest pain, anorexia, rash, and/or abdominal pain. Additional testing may be needed to monitor or reduce the likelihood of adverse effects.
Compliance with Treatment
Advise patients to take SIRTURO in combination with other antimycobacterial drugs as prescribed. Emphasize compliance with the full course of therapy. Advise patients that skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the treatment and (2) increase the likelihood that their mycobacterium may develop resistance and the disease will not be treatable by SIRTURO or other antibacterial drugs in the future.
If a dose is missed during the first 2 weeks of treatment, advise patients not to make up the missed dose but to continue the usual dosing schedule. From Week 3 onwards, if a 200 mg dose is missed, advise patients to take the missed dose as soon as possible, and then resume the 3 times a week regimen.
Use with Alcohol and other Medications
Advise patients to abstain from alcohol, hepatotoxic medications or herbal products.
Advise patients to discuss with their physician the other medications they are taking and other medical conditions before starting treatment with SIRTURO.
Lactation
Advise patients or caregivers to monitor infants exposed to bedaquiline through breast milk for signs of bedaquiline-related adverse reactions, such as hepatotoxicity (yellowing of the eyes and changes in the color of the urine or stool) [see Adverse Reactions (6) and Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 8/2019 MEDICATION GUIDE
SIRTURO® (ser toor' oh)
(bedaquiline)
Tablets, for oral useRead this Medication Guide before you start taking SIRTURO® and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. What is the most important information I should know about SIRTURO?
SIRTURO can cause serious side effects, including:- Increased risk of death. Some people who had pulmonary tuberculosis resistant to other antibiotics (multi-drug resistant tuberculosis) and were treated with SIRTURO, had an increased risk in death.
- A serious heart rhythm problem called QT prolongation. This condition can cause an abnormal heartbeat in people who take SIRTURO and may lead to death. Your healthcare provider should check your heart and do blood tests before and during treament with SIRTURO. Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat) or if you feel dizzy or faint.
What is SIRTURO?
SIRTURO is a diarylquinoline antibiotic prescription medicine used in people 12 years of age and older with multi-drug resistant tuberculosis (MDR-TB) of the lungs when other effective treatment options are not possible.
It is not known if SIRTURO is safe and effective in:- people who have a tuberculosis (TB) infection, but do not show symptoms of TB (also known as latent TB).
- people who have TB that is not resistant to antibiotics.
- people who have types of TB other than TB of the lungs.
- people who have an infection caused by a bacteria other than TB.
- people who are being treated for Human Immunodeficiency Virus (HIV) who also have MDR-TB.
- children under 12 years of age or weighing less than 66 pounds (30 kg).
Before you take SIRTURO, tell your healthcare provider about all your medical conditions including, if you: - take any other medicines for your heart.
- have had an abnormal heart rhythm (ECG) or other heart problems.
- have a family history of a heart problem called "congenital long QT syndrome" or heart failure.
- have decreased thyroid gland function (hypothyroidism).
- have liver or kidney problems.
- have HIV infection.
- are pregnant or plan to become pregnant. It is not known if SIRTURO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SIRTURO passes into breast milk. Talk to your healthcare provider about the best way to feed your baby while taking SIRTURO.
- If you and your healthcare provider decide for you to breastfeed while taking SIRTURO, tell your healthcare provider right away if your baby has:
- yellowing of their eyes.
- darker than usual urine color.
- lighter than usual stool color or stool that is pale or light brown.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
You should not take certain liver medicines or herbal supplements while taking SIRTURO.How should I take SIRTURO? - Take SIRTURO exactly as your healthcare provider tells you to take it.
- You will take SIRTURO for a total of 24 weeks. You may need to take your other TB medicines for longer than 24 weeks. If you are not sure, you should talk with your healthcare provider.
- SIRTURO must always be taken with other medicines to treat TB. Your healthcare provider will decide which other medicines you should take with SIRTURO.
- It is important that you complete the full course of treatment with SIRTURO and not skip doses. Skipping doses may decrease the effectiveness of the treatment and increase the chances that your TB will not be treatable by SIRTURO or other medicines.
- Take SIRTURO with food. Swallow the tablets whole with water.
Take 400 mg (4 tablets) 1 time each day.
Week 3 to Week 24:- Take 200 mg (2 tablets) a day 3 times a week.
- Take SIRTURO doses at least 48 hours apart. For example, you may take SIRTURO on Monday, Wednesday and Friday every week from week 3 to week 24.
- Do not skip SIRTURO doses. If you skip doses, or do not complete the total 24 weeks of SIRTURO, your treatment may not work as well, and your TB may be harder to treat.
- If you take more SIRTURO than you should, talk to a healthcare provider right away.
If you miss your SIRTURO dose during Week 1 or Week 2: - Do not take a double dose to make up for the missed dose. Take the next dose as usual.
If you miss your SIRTURO dose during Week 3 to Week 24: - Take the missed dose as soon as possible and continue taking SIRTURO on the 3 times a week schedule.
- If you miss a dose and you are not sure what to do, talk to your healthcare provider.
- Do not stop taking SIRTURO without first talking to your healthcare provider.
What should I avoid while taking SIRTURO? - You should not drink alcohol while taking SIRTURO.
What are the possible side effects of SIRTURO?
SIRTURO may cause serious side effects, including:- See "What is the most important information I should know about SIRTURO?"
- liver problems (hepatotoxicity). Call your healthcare provider right away if you have unexplained symptoms such as nausea or vomiting, stomach pain, fever, weakness, itching, unusual tiredness, loss of appetite, light colored bowel movements, dark colored urine, yellowing of your skin or the white of your eyes.
The most common side effects of SIRTURO in children include joint pain, nausea and stomach pain.
These are not all the possible side effects of SIRTURO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store SIRTURO? - Store SIRTURO at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SIRTURO in the original container, and keep SIRTURO out of light.
General information about the safe and effective use of SIRTURO:
This Medication Guide summarizes the most important information about SIRTURO. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about SIRTURO that is written for health professionals.What are the ingredients in SIRTURO?
Active ingredient: bedaquiline
Inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, purified water (removed during processing)
Product of India
Finished Product Manufactured by: Recipharm Pharmaservices Pvt. Ltd., Bangalore, India
Manufactured for: Janssen Therapeutics, Division of Janssen Products, LP Titusville, NJ 08560
© Janssen Products, LP 2012 -
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
NDC: 59676-701-01
Sirturo®
(bedaquiline) tablets100 mg
Dispense Medication Guide
to each patientAttention Pharmacist: Dispense in original
container. Tablets dispensed outside the
original container should be stored in a tight
light-resistant container with an expiration
date not to exceed 3 months. Store at 25°C
(77°F); Excursions permitted to 15°C-30°C
(59°F - 86°F).[See USP Controlled Room
Temperature].188 Tablets
Rx only
janssen
-
INGREDIENTS AND APPEARANCE
SIRTURO
bedaquiline fumarate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-701 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bedaquiline Fumarate (UNII: P04QX2C1A5) (Bedaquiline - UNII:78846I289Y) Bedaquiline 100 mg Inactive Ingredients Ingredient Name Strength starch, corn (UNII: O8232NY3SJ) silicon dioxide (UNII: ETJ7Z6XBU4) croscarmellose sodium (UNII: M28OL1HH48) hypromellose 2910 (15 mPa.s) (UNII: 36SFW2JZ0W) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) polysorbate 20 (UNII: 7T1F30V5YH) water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score no score Shape ROUND (round, biconvex) Size 11mm Flavor Imprint Code T;207;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-701-01 188 in 1 BOTTLE; Type 0: Not a Combination Product 12/28/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204384 12/28/2012 Labeler - Janssen Products, LP (804684207) Establishment Name Address ID/FEI Business Operations Dishman Carbogen Amcis Limited 915628142 API MANUFACTURE(59676-701) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 400345889 API MANUFACTURE(59676-701) Establishment Name Address ID/FEI Business Operations Recipharm Pharma Services Private Limited 871401927 MANUFACTURE(59676-701) , ANALYSIS(59676-701)
Trademark Results [SIRTURO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIRTURO 85698237 4389452 Live/Registered |
JOHNSON & JOHNSON 2012-08-08 |
 SIRTURO 78579594 not registered Dead/Abandoned |
Johnson & Johnson 2005-03-03 |
 SIRTURO 77728865 not registered Dead/Abandoned |
JOHNSON & JOHNSON 2009-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.