ZYLOTROL PAIN RELIEVING- lidocaine hcl, menthol cream
ZYLOTROL PAIN RELIEVING by
Drug Labeling and Warnings
ZYLOTROL PAIN RELIEVING by is a Otc medication manufactured, distributed, or labeled by Asclemed USA, Inc., ASCLEMED USA INC. DBA ENOVACHEM. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredients
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
Ask a doctor before use ifyou have a heart condition.
Do not use if you are allergic to any other type of numbing medicine in large quantities, particularly over raw surfaces or blistered areas on infections on deep puncture wounds if pregnant or breastfeeding.
When using this product do not use over large skin areas do not apply heat, bandages or plastic wrap to treated areas do not use in or near the eyes wash hands immediately after using.
Stop use and ask a doctor if allergic reaction occurs condition worsens or does not improve within 7 days symptoms clear up and return within a few days redness, irritation, swelling, pain or other symptoms begin or increase.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients:
Allantoin, Aloe Barbadensis Leaf Juice*, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Arnica Montana Flower Extract, Behenyl Alcohol, Boswellia Serrata Extract, Butyrospermum Parkii (Shea) Butter, Calophyllum Inophyllum (Tamanu) Seed Oil, Caprylic/capric Triglyceride, Ceteareth-20, Cetearyl Alcohol, Chamomilla Recutita (Matricaria) Flower Extract, Ethylhexylglycerin, Hypericum Perforatum (St. John's Wort) Flower/leaf/stem Extract, Glyceryl Stearate, Glycerin, Hydrogenated Polydecene, Isopropyl Palmitate, Peg-100 Stearate, Persea Gratissima (Avocado) Oil, Phenoxyethanol, Polysorbate 60, Propanediol, Sodium Polyacrylate, Stearyl Alcohol, Tetrahydroxypropyl Ethylenediamine, Trideceth-6, Water.
*Certified Organic Ingredient - Mayacert Certifier
- QUESTIONS
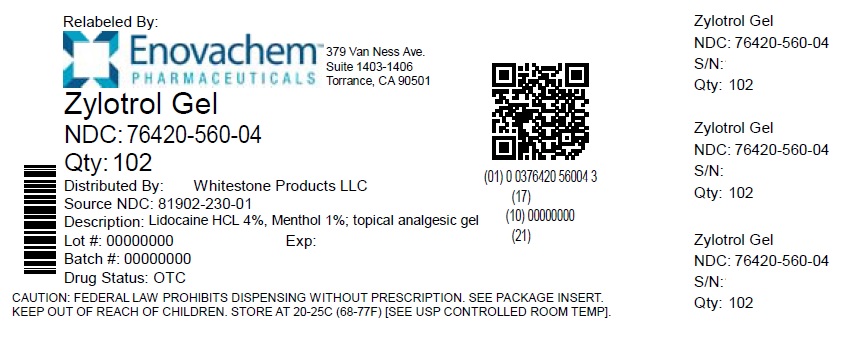
- Label
-
INGREDIENTS AND APPEARANCE
ZYLOTROL PAIN RELIEVING
lidocaine hcl, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76420-560(NDC:81902-230) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) SHEA BUTTER (UNII: K49155WL9Y) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DOCOSANOL (UNII: 9G1OE216XY) EDETOL (UNII: Q4R969U9FR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ALLANTOIN (UNII: 344S277G0Z) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) AVOCADO OIL (UNII: 6VNO72PFC1) TAMANU OIL (UNII: JT3LVK84A1) POLYSORBATE 60 (UNII: CAL22UVI4M) ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CHAMOMILE (UNII: FGL3685T2X) ST. JOHN'S WORT (UNII: UFH8805FKA) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) TRIDECETH-6 (UNII: 3T5PCR2H0C) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76420-560-04 102 g in 1 TUBE; Type 0: Not a Combination Product 05/15/2023 2 NDC: 76420-560-02 102 g in 1 JAR; Type 0: Not a Combination Product 10/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/29/2022 Labeler - Asclemed USA, Inc. (059888437) Establishment Name Address ID/FEI Business Operations ASCLEMED USA INC. DBA ENOVACHEM 059888437 relabel(76420-560)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.