Fordagel Kit by Medcore LLC Fordagel Kit Drug Facts
Fordagel Kit by
Drug Labeling and Warnings
Fordagel Kit by is a Otc medication manufactured, distributed, or labeled by Medcore LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
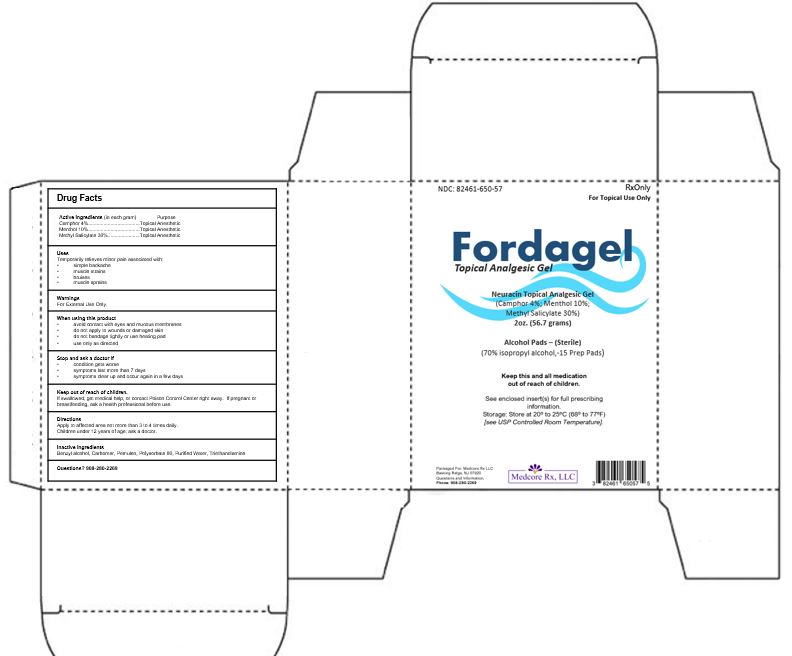
FORDAGEL KIT- neuracin topical analgesic gel gel
Medcore LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Fordagel Kit
Drug Facts
Uses
Temporarily relieves minor pain associated with:
- simple backache
- muscle strains
- bruises
- muscle sprains
Warnings
For External Use Only.
When using this product
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly or use heating pad
- use only as directed
Stop and ask a doctor if
- condition gets worse
- symptoms last more than 7 days
- symptoms clear up and occur again in a few days
Keep out of reach of children.
If swallowed, get medical help, or contact Poison Control Center right away. If pregnant or breastfeeding, ask a health professional before use.
Directions
Apply to affected area not more than 3 to 4 times daily.
Children under 12 years of age: ask a doctor.
Inactive Ingredients
Benzyl alcohol, Carbomer, Pemulen, Polysorbate 80, Purified Water, Triethanolamine
Questions? 908-280-2269
NDC: 82461-650-57
RxOnly
For Topical Use Only
Fordagel
Topical Analgesic Gel
Neuracin Topical Analgesic Gel
(Camphor 4%; Menthol 10%;
Methyl Salicylate 30%)
2oz. (56.7 grams)
Alcohol Pads – (Sterile)
(70% isopropyl alcohol,-15 Prep Pads)
Keep this and all medication
out of reach of children.
See enclosed insert(s) for full prescribing information.
Storage: Store at 20º to 25ºC (68º to 77ºF)
[see USP Controlled Room Temperature].
| FORDAGEL KIT
neuracin topical analgesic gel gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Medcore LLC (069802634) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.