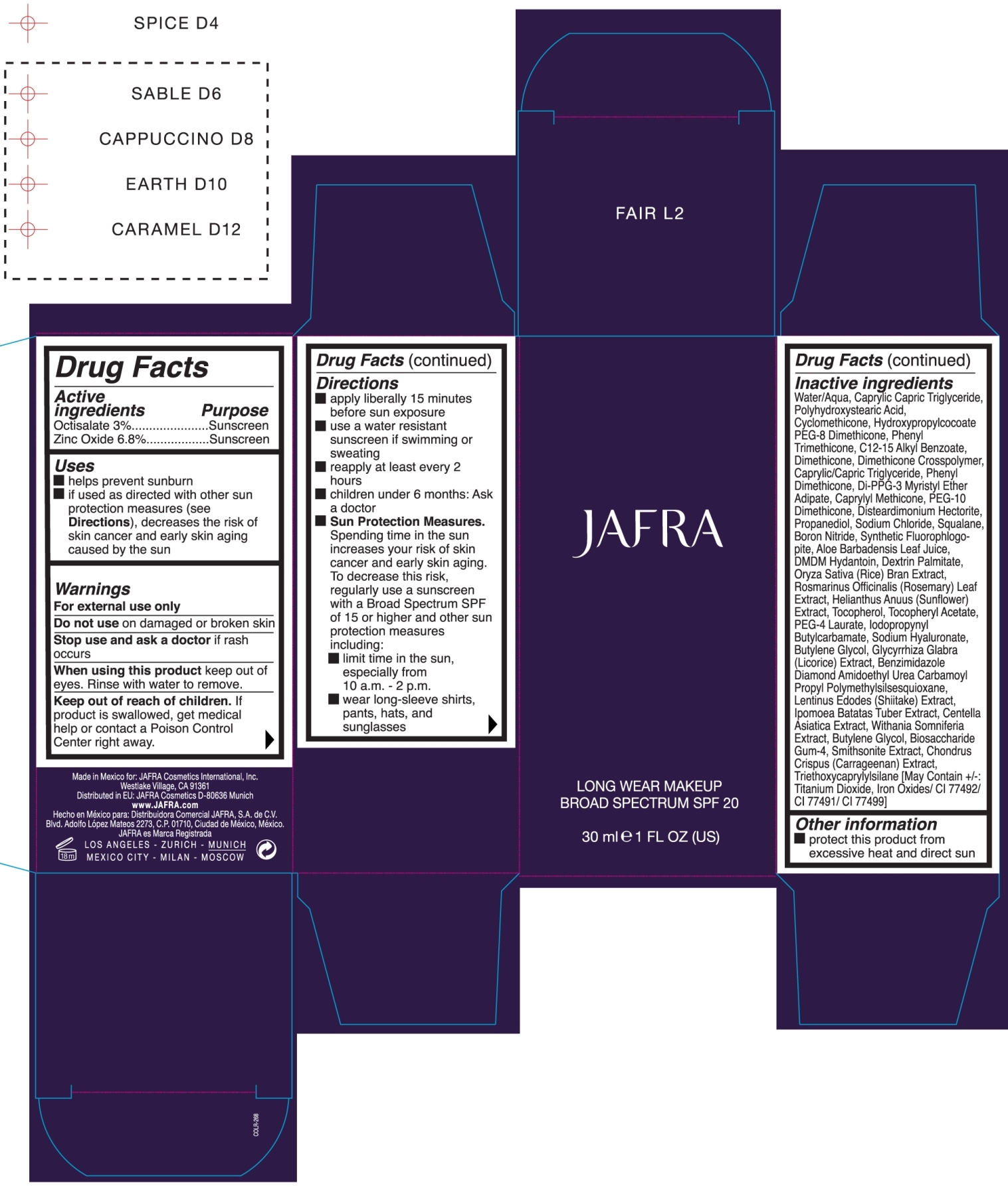
LONG WEAR MAKEUP BROAD SPECTRUM SPF 20 CAPPUCCINO JAFRA- octisalate, zinc oxide cream
Long Wear Makeup Broad Spectrum SPF 20 Cappuccino by
Drug Labeling and Warnings
Long Wear Makeup Broad Spectrum SPF 20 Cappuccino
by is a Otc medication manufactured, distributed, or labeled by JAFRA COSMETICS INTERNATIONAL, JAFRA COSMETICS INTERNATIONAL ,
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
INACTIVE INGREDIENT
Water/Aqua, Caprylic Capric Triglyceride, Polyhydroxystearic Acid, Cyclomethicone, Hydroxypropylcocoate PEG-8 Dimethicone, Phenyl Triemthicone, C12-15 Alkyl Benzoate, Dimethicone, Dimethicone Crosspolymer, Caprylic/Capric Triglyceride, Phenyl Dimethicone, Di-PPG-3 Myristyl Ether Adipate, Caprylyl Methicone, PEG-10 Dimethicone, Disteardimonium Hectorite, Propanediol, Sodium Chloride, Squalane,Boron Nitride, Synthetic Fluorophlogopite, Aloe Barbadensis Leaf Juice, DMDM Hydantoin, Dextrin Palmitate, Oryza Sativa (Rice) Bran Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Helianthus Anuus (Sunflower) Extract, Tocopherol, Tocopheryl Acetate, PEG-4 Laurate, Iodopropynyl Butylcarbamate, Sodium Hyaluronate, Butylene Glycol, Glycyrrhiza Glabra (Licorice) Extract, Benzimidazole Diamond Amidoethyl Urea Carbamoyl Propyl Polymehtylsilsesquioxane, Lentinus Edodes (shiitake) Extract, Ipomoea Batatas Tuber Extract, Centella Asiatica Extract, Withania Somniferia Extract, Butylene Glycol, Biosaccharide Gum-4, Smithsonite Extact, Chondrus Crispus (Carrageenan) Extract, Triethoxycaprylylsilane [May Containt +/-: Titanium Dioxide, Iron Oxides/ CI 77492/ CI 77491/ CI 77499]
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LONG WEAR MAKEUP BROAD SPECTRUM SPF 20 CAPPUCCINO JAFRA
octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68828-271 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 6.8 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CYCLOMETHICONE (UNII: NMQ347994Z) HYDROXYPROPYLCOCOATE PEG-8 DIMETHICONE (UNII: 8TE0BZU36S) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DI-PPG-3 MYRISTYL ETHER ADIPATE (UNII: T32481VTXW) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPANEDIOL (UNII: 5965N8W85T) SODIUM CHLORIDE (UNII: 451W47IQ8X) SQUALANE (UNII: GW89575KF9) BORON NITRIDE (UNII: 2U4T60A6YD) ALOE VERA LEAF (UNII: ZY81Z83H0X) DMDM HYDANTOIN (UNII: BYR0546TOW) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) RICE BRAN (UNII: R60QEP13IC) ROSEMARY (UNII: IJ67X351P9) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PEG-4 LAURATE (UNII: AYF4VM3N1Z) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LICORICE (UNII: 61ZBX54883) LENTINULA EDODES MYCELIUM (UNII: CU2S39TB8O) SWEET POTATO (UNII: M9WGG9Z9GK) CENTELLA ASIATICA (UNII: 7M867G6T1U) WITHANIA SOMNIFERA LEAF (UNII: J337CNM3CW) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68828-271-02 1 in 1 BOX 02/21/2017 1 NDC: 68828-271-01 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/21/2017 Labeler - JAFRA COSMETICS INTERNATIONAL (041676479) Registrant - JAFRA COSMETICS INTERNATIONAL (041676479) Establishment Name Address ID/FEI Business Operations <text xmlns="urn:hl7-org:v3"> <paragraph> <content styleCode="bold">Active </content> </paragraph> <paragraph> <content styleCode="bold">Ingredients Purpose</content> </paragraph> <paragraph>Octisalate 3% ...... Sunscreen</paragraph> <paragraph>Zinc Oxide 6.8% .... Sunscreen</paragraph> <paragraph/></text> 814732061 manufacture(68828-271)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.