DOXORUBICIN HYDROCHLORIDE injectable, liposomal
DOXORUBICIN HYDROCHLORIDE by
Drug Labeling and Warnings
DOXORUBICIN HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals USA Inc., Zydus Lifesciences Global FZE, Zydus Lifesciences Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DOXORUBICIN HYDROCHLORIDE LIPOSOME INJECTION safely and effectively. See full prescribing information for DOXORUBICIN HYDROCHLORIDE LIPOSOME INJECTION.
DOXORUBICIN HYDROCHLORIDE LIPOSOME injection, for intravenous use
Initial U.S. Approval: 1995WARNING: CARDIOMYOPATHY and INFUSION-RELATED REACTIONS
See full prescribing information for complete boxed warning.
- Doxorubicin hydrochloride liposome injection can cause myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy was 11% when the cumulative anthracycline dose was between 450 mg/m2to 550 mg/m2. Assess left ventricular cardiac function prior to initiation of doxorubicin hydrochloride liposome injection, during treatment and after treatment (5.1).
- Serious, life-threatening, and fatal infusion-related reactions can occur with doxorubicin hydrochloride liposome injection. Acute infusion-related reactions occurred in 11% of patients with solid tumors. Withhold doxorubicin hydrochloride liposome injection for infusion-related reactions and resume at a reduced rate. Discontinue doxorubicin hydrochloride liposome injection for serious or life-threatening infusion-related reactions (5.2).
INDICATIONS AND USAGE
Doxorubicin hydrochloride liposome injection is an anthracycline topoisomerase inhibitor indicated for:
- Ovarian cancer: After failure of platinum-based chemotherapy (1.1)
- AIDS-related Kaposi's Sarcoma: After failure of prior systemic chemotherapy or intolerance to such therapy (1.2)
- Multiple Myeloma: In combination with bortezomib in patients who have not previously received bortezomib and have received at least one prior therapy (1.3)
DOSAGE AND ADMINISTRATION
Administer doxorubicin hydrochloride liposome injection at an initial rate of 1 mg/min to minimize the risk of infusion reactions. If no infusion related reactions occur, increase rate of infusion to complete administration over 1 hour. Do not administer as bolus injection or undiluted solution (2).
DOSAGE FORMS AND STRENGTHS
Doxorubicin hydrochloride liposome injection: Single dose vials: 20 mg/10 mL and 50 mg/25 mL (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (>20%) are asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia (6).
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Discontinue breastfeeding (8.2).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
BOXED WARNING
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
1.2 AIDS-Related Kaposi's Sarcoma
1.3 Multiple Myeloma
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
2.2 Ovarian Cancer
2.3 AIDS-Related Kaposi's Sarcoma
2.4 Multiple Myeloma
2.5 Dose Modifications for Adverse Reactions
2.6 Preparation and Administration
2.7 Procedure for Proper Handling and Disposal
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
5.2 Infusion-Related Reactions
5.3 Hand-Foot Syndrome (HFS)
5.4 Secondary Oral Neoplasms
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ovarian Cancer
14.2 AIDS-Related Kaposi's Sarcoma
14.3 Multiple Myeloma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: CARDIOMYOPATHY and INFUSION-RELATED REACTIONS
- Doxorubicin hydrochloride liposome injection can cause myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy was 11% when the cumulative anthracycline dose was between 450 mg/m2to 550 mg/m2. Assess left ventricular cardiac function prior to initiation of doxorubicin hydrochloride liposome injection and during and after treatment [see Warnings and Precautions (5.1)].
- Serious, life-threatening, and fatal infusion-related reactions can occur with doxorubicin hydrochloride liposome injection. Acute infusion-related reactions occurred in 11% of patients with solid tumors. Withhold doxorubicin hydrochloride liposome injection for infusion-related reactions and resume at a reduced rate. Discontinue doxorubicin hydrochloride liposome injection for serious or life-threatening infusion-related reactions [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
Doxorubicin hydrochloride liposome injection is indicated for the treatment of patients with ovarian cancer whose disease has progressed or recurred after platinum-based chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
Do not substitute doxorubicin hydrochloride liposome injection for other doxorubicin hydrochloride products.
Do not administer as an undiluted suspension or as an intravenous bolus [see Warnings and Precautions (5.2)].
2.2 Ovarian Cancer
The recommended dose of doxorubicin hydrochloride liposome injection is 50 mg/m2 intravenously over 60 minutes every 28 days until disease progression or unacceptable toxicity.
2.3 AIDS-Related Kaposi's Sarcoma
The recommended dose of doxorubicin hydrochloride liposome injection is 20 mg/m2 intravenously over 60 minutes every 21 days until disease progression or unacceptable toxicity.
2.4 Multiple Myeloma
The recommended dose of doxorubicin hydrochloride liposome injection is 30 mg/m2 intravenously over 60 minutes on day 4 of each 21-day cycle for eight cycles or until disease progression or unacceptable toxicity. Administer doxorubicin hydrochloride liposome injection after bortezomib on day 4 of each cycle [see Clinical Studies (14.3)].
2.5 Dose Modifications for Adverse Reactions
Do not increase doxorubicin hydrochloride liposome injection after a dose reduction for toxicity.
Table 1: Recommended Dose Modifications for Hand-Foot Syndrome, Stomatitis, or Hematologic Adverse Reactions Toxicity
Dose Adjustment
Hand-Foot Syndrome (HFS)
Grade 1: Mild erythema, swelling, or desquamation not interfering with daily activities
- If no previous Grade 3 or 4 HFS: no dose adjustment.
- If previous Grade 3 or 4 HFS: delay dose up to 2 weeks, then decrease dose by 25%.
Grade 2: Erythema, desquamation, or swelling interfering with, but not precluding normal physical activities; small blisters or ulcerations less than 2 cm in diameter
- Delay dosing up to 2 weeks or until resolved to Grade 0-1.
- Discontinue doxorubicin hydrochloride liposome injection if no resolution after 2 weeks.
- If resolved to Grade 0-1 within 2 weeks:
o And previous Grade 3 or 4 toxicity: decrease dose by 25%.
Grade 3: Blistering, ulceration, or swelling interfering with walking or normal daily activities; cannot wear regular clothing
- Delay dosing up to 2 weeks or until resolved to Grade 0-1, then decrease dose by 25%.
- Discontinue doxorubicin hydrochloride liposome injection if no resolution after 2 weeks.
Grade 4: Diffuse or local process causing infectious complications, or a bed ridden state or hospitalization
- Delay dosing up to 2 weeks or until resolved to Grade 0-1, then decrease dose by 25%.
- Discontinue doxorubicin hydrochloride liposome injection if no resolution after 2 weeks.
Stomatitis
Grade 1: Painless ulcers, erythema, or mild soreness
- If no previous Grade 3 or 4 toxicity: no dose adjustment.
- If previous Grade 3 or 4 toxicity: delay up to 2 weeks then decrease dose by 25%.
Grade 2: Painful erythema, edema, or ulcers, but can eat
- Delay dosing up to 2 weeks or until resolved to Grade 0-1.
- Discontinue doxorubicin hydrochloride liposome injection if there is no resolution after 2 weeks.
- If resolved to Grade 0-1 within 2 weeks:
o And previous Grade 3 or 4 toxicity: decrease dose by 25%.
Grade 3: Painful erythema, edema, or ulcers, and cannot eat
- Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to original dose interval.
- If after 2 weeks there is no resolution, discontinue doxorubicin hydrochloride liposome injection.
Grade 4: Requires parenteral or enteral support
- Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to original dose interval.
- If after 2 weeks there is no resolution, discontinue doxorubicin hydrochloride liposome injection.
Neutropenia or Thrombocytopenia
Grade 1
No dose reduction
Grade 2
Delay until ANC ≥ 1,500 and platelets ≥ 75,000; resume treatment at previous dose
Grade 3
Delay until ANC ≥ 1,500 and platelets ≥ 75,000; resume treatment at previous dose
Grade 4
Delay until ANC ≥ 1,500 and platelets ≥ 75,000; resume at 25% dose reduction or continue previous dose with prophylactic granulocyte growth factor
Table 2: Recommended Dose Modifications of Doxorubicin Hydrochloride Liposome Injection for Toxicity When Administered in Combination With Bortezomib Toxicity
Doxorubicin Hydrochloride Liposome Injection
Fever ≥38°C and ANC <1,000/mm3
- Withhold dose for this cycle if before Day 4;
- Decrease dose by 25%, if after Day 4 of previous cycle.
On any day of drug administration after Day 1 of each cycle:
- Platelet count <25,000/mm3
- Hemoglobin <8 g/dL
- ANC <500/mm3
- Withhold dose for this cycle if before Day 4;
- Decrease dose by 25%, if after Day 4 of previous cycle AND if bortezomib is reduced for hematologic toxicity.
Grade 3 or 4 non-hematologic drug related toxicity
Do not dose until recovered to Grade <2, then reduce dose by 25%.
For neuropathic pain or peripheral neuropathy, no dosage adjustments are required for doxorubicin hydrochloride liposome injection. Refer to bortezomib manufacturer's prescribing information.
2.6 Preparation and Administration
Dilute doxorubicin hydrochloride liposome injection doses up to 90 mg in 250 mL of 5% Dextrose Injection, USP prior to administration. Dilute doses exceeding 90 mg in 500 mL of 5% Dextrose Injection, USP prior to administration. Refrigerate diluted doxorubicin hydrochloride liposome injection at 2°C to 8°C (36°F to 46°F) and administer within 24 hours.
Administration
Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if a precipitate or foreign matter is present.
Do not use with in-line filters.
Administer the first dose of doxorubicin hydrochloride liposome injection at an initial rate of 1 mg/min. If no infusion-related adverse reactions are observed, increase the infusion rate to complete the administration of the drug over one hour [see Warnings and Precautions (5.2)]. Do not rapidly flush the infusion line.
Do not mix doxorubicin hydrochloride liposome injection with other drugs.
Management of Suspected Extravasation
Discontinue doxorubicin hydrochloride liposome injection for burning or stinging sensation or other evidence indicating perivenous infiltration or extravasation. Manage confirmed or suspected extravasation as follows:
- Do not remove the needle until attempts are made to aspirate extravasated fluid
- Do not flush the line
- Avoid applying pressure to the site
- Apply ice to the site intermittently for 15 minutes 4 times a day for 3 days
- If the extravasation is in an extremity, elevate the extremity
2.7 Procedure for Proper Handling and Disposal
Doxorubicin hydrochloride liposome injection is a cytotoxic drug. Follow applicable special handling and disposal procedures.1 If doxorubicin hydrochloride liposome injection comes into contact with skin or mucosa, immediately wash thoroughly with soap and water.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Doxorubicin hydrochloride liposome injection is contraindicated in patients who have a history of severe hypersensitivity reactions, including anaphylaxis, to doxorubicin hydrochloride [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
Doxorubicin hydrochloride can cause myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy with doxorubicin hydrochloride is generally proportional to the cumulative exposure. Include prior use of other anthracyclines or anthracenediones in calculations of cumulative dose. The risk of cardiomyopathy may be increased at lower cumulative doses in patients with prior mediastinal irradiation.
In a clinical study in 250 patients with advanced cancer who were treated with doxorubicin hydrochloride liposome injection, the risk of cardiomyopathy was 11% when the cumulative anthracycline dose was between 450 mg/m2to 550 mg/m2. Cardiomyopathy was defined as >20% decrease in resting left ventricular ejection fraction (LVEF) from baseline where LVEF remained in the normal range or a >10% decrease in LVEF from baseline where LVEF was less than the institutional lower limit of normal. Two percent of patients developed signs and symptoms of congestive heart failure without documented evidence of cardiomyopathy.
Assess left ventricular cardiac function (e.g. MUGA or echocardiogram) prior to initiation of doxorubicin hydrochloride liposome injection, during treatment to detect acute changes, and after treatment to detect delayed cardiomyopathy. Administer doxorubicin hydrochloride liposome injection to patients with a history of cardiovascular disease only when the potential benefit of treatment outweighs the risk.
5.2 Infusion-Related Reactions
Serious, life-threatening, and fatal infusion-related reactions characterized by one or more of the following symptoms can occur with doxorubicin hydrochloride liposome injection: flushing, shortness of breath, facial swelling, headache, chills, chest pain, back pain, tightness in the chest and throat, fever, tachycardia, pruritus, rash, cyanosis, syncope, bronchospasm, asthma, apnea, and hypotension. Of 239 patients with ovarian cancer treated with doxorubicin hydrochloride liposome injection in Trial 4, 7% of patients experienced acute infusion-related reactions resulting in dose interruption. All occurred during cycle 1 and none during subsequent cycles. Across multiple studies of doxorubicin hydrochloride liposome injection monotherapy including this and other studies enrolling 760 patients with various solid tumors, 11% of patients had infusion-related reactions. The majority of infusion-related events occurred during the first infusion.
Ensure that medications to treat infusion-related reactions and cardiopulmonary resuscitative equipment are available for immediate use prior to initiation of doxorubicin hydrochloride liposome injection. Initiate doxorubicin hydrochloride liposome injections at a rate of 1 mg/min and increase rate as tolerated [see Dosage and Administration (2.6)]. Withhold doxorubicin hydrochloride liposome injection for Grade 1, 2, or 3 infusion-related reactions and resume at a reduced infusion rate. Discontinue doxorubicin hydrochloride liposome injection for serious or life-threatening infusion-related reactions.
5.3 Hand-Foot Syndrome (HFS)
In Trial 4, the incidence of HFS was 51% of patients in the doxorubicin hydrochloride liposome injection arm and 0.9% of patients in the topotecan arm, including 24% Grade 3 or 4 cases of HFS in doxorubicin hydrochloride liposome injection-treated patients and no Grade 3 or 4 cases in topotecan-treated patients. HFS or other skin toxicity required discontinuation of doxorubicin hydrochloride liposome injection in 4.2% of patients.
HFS was generally observed after 2 or 3 cycles of treatment but may occur earlier. Delay doxorubicin hydrochloride liposome injection for the first episode of Grade 2 or greater HFS [see Dosage and Administration (2.5)]. Discontinue doxorubicin hydrochloride liposome injection if HFS is severe and debilitating.
5.4 Secondary Oral Neoplasms
Secondary oral cancers, primarily squamous cell carcinoma, have been reported from post-marketing experience in patients with long-term (more than one year) exposure to doxorubicin hydrochloride liposome injection. These malignancies were diagnosed both during treatment with doxorubicin hydrochloride liposome injection and up to 6 years after the last dose. Examine patients at regular intervals for the presence of oral ulceration or with any oral discomfort that may be indicative of secondary oral cancer.
The altered pharmacokinetics and preferential tissue distribution of liposomal doxorubicin that contributes to enhanced skin toxicity and mucositis compared to free doxorubicin may play a role in the development of oral secondary malignancies with long-term use.
5.5 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, doxorubicin hydrochloride liposome injection can cause fetal harm when administered to a pregnant woman; avoid the use of doxorubicin hydrochloride liposome injection during the 1st trimester. Available human data do not establish the presence or absence of major birth defects and miscarriage related to the use of doxorubicin hydrochloride during the 2nd and 3rd trimesters. At doses approximately 0.12 times the recommended clinical dose, doxorubicin hydrochloride liposome injection was embryotoxic and abortifacient in rabbits. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during and for 6 months after treatment with doxorubicin hydrochloride liposome injection [see Use in Specific Populations (8.1), (8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling.
- Cardiomyopathy [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Hand-Foot Syndrome [see Warnings and Precautions (5.3)]
- Secondary Oral Neoplasms [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates on other clinical trials and may not reflect the rates observed in clinical practice.
The safety data reflect exposure to doxorubicin hydrochloride liposome injection in 1310 patients including: 239 patients with ovarian cancer, 753 patients with AIDS-related Kaposi's sarcoma, and 318 patients with multiple myeloma. The most common adverse reactions (>20%) observed with doxorubicin hydrochloride liposome injection are asthenia, fatigue, fever, nausea, stomatitis, vomiting, diarrhea, constipation, anorexia, hand-foot syndrome, rash and neutropenia, thrombocytopenia and anemia.
The following tables present adverse reactions from clinical trials of single-agent doxorubicin hydrochloride liposome injection in ovarian cancer and AIDS-Related Kaposi's sarcoma.
Patients With Ovarian Cancer
The safety data described below are from Trial 4, which included 239 patients with ovarian cancer treated with doxorubicin hydrochloride liposome injection 50 mg/m2 once every 4 weeks for a minimum of four courses in a randomized, multicenter, open-label study. In this trial, patients received doxorubicin hydrochloride liposome injection for a median number of 3.2 months (range 1 day to 25.8 months). The median age of the patients is 60 years (range 27 to 87), with 91% Caucasian, 6% Black, and 3% Hispanic or Other.
Table 3 presents the hematologic adverse reactions from Trial 4.
Table 3: Hematologic Adverse Reactions in Trial 4 Doxorubicin Hydrochloride Liposome Injection Patients
(n=239)
Topotecan Patients
(n=235)
Neutropenia
500 - <1,000/mm3
8%
14%
<500/mm3
4.2%
62%
Anemia
6.5 - <8 g/dL
5%
25%
<6.5 g/dL
0.4%
4.3%
Thrombocytopenia
10,000 - <50,000/mm3
1.3%
17%
<10,000/mm3
0.0%
17%
Table 4 presents the non-hematologic adverse reactions from Trial 4.
Table 4: Non-Hematologic Adverse Reactions in Trial 4 Non-Hematologic
Adverse Reaction
10% or Greater
Doxorubicin Hydrochloride Liposome Injection (%)
Treated
(n=239)
Topotecan (%)
Treated
(n=235)
All grades
Grades 3-4
All grades
Grades 3-4
Body as a Whole
Asthenia
40
7
52
8
Fever
21
0.8
31
6
Mucous Membrane Disorder
14
3.8
3.4
0
Back Pain
12
1.7
10
0.9
Infection
12
2.1
6
0.9
Headache
11
0.8
15
0
Digestive
Nausea
46
5
63
8
Stomatitis
41
8
15
0.4
Vomiting
33
8
44
10
Diarrhea
21
2.5
35
4.2
Anorexia
20
2.5
22
1.3
Dyspepsia
12
0.8
14
0
Nervous
Dizziness
4.2
0
10
0
Respiratory
Pharyngitis
16
0
18
0.4
Dyspnea
15
4.1
23
4.3
Cough increased
10
0
12
0
Skin and Appendages
Hand-foot syndrome
51
24
0.9
0
Rash
29
4.2
12
0.4
Alopecia
19
N/A
52
N/A
The following additional adverse reactions were observed in patients with ovarian cancer with doses administered every four weeks (Trial 4).
Incidence 1% to 10%
Cardiovascular: vasodilation, tachycardia, deep vein thrombosis, hypotension, cardiac arrest.
Digestive: oral moniliasis, mouth ulceration, esophagitis, dysphagia, rectal bleeding, ileus.
Hematologic and Lymphatic: ecchymosis.
Metabolic and Nutritional: dehydration, weight loss, hyperbilirubinemia, hypokalemia, hypercalcemia, hyponatremia.
Nervous: somnolence, dizziness, depression.
Respiratory: rhinitis, pneumonia, sinusitis, epistaxis.
Skin and Appendages: pruritus, skin discoloration, vesiculobullous rash, maculopapular rash, exfoliative dermatitis, herpes zoster, dry skin, herpes simplex, fungal dermatitis, furunculosis, acne.
Special Senses: conjunctivitis, taste perversion, dry eyes.
Urinary: urinary tract infection, hematuria, vaginal moniliasis.
Patients With AIDS-Related Kaposi's Sarcoma
The safety data described is based on the experience reported in 753 patients with AIDS-related Kaposi's sarcoma (KS) enrolled in four open-label, uncontrolled trials of doxorubicin hydrochloride liposome injection administered at doses ranging from 10 to 40 mg/m2 every 2 to 3 weeks. Demographics of the population were: median age 38.7 years (range 24-70); 99% male; 88% Caucasian, 6% Hispanic, 4% Black, and 2% Asian/other/unknown. The majority of patients were treated with 20 mg/m2 of doxorubicin hydrochloride liposome injection every 2 to 3 weeks with a median exposure of 4.2 months (range 1 day to 26.6 months). The median cumulative dose was 120 mg/m2 (range 3.3 to 798.6 mg/m2); 3% received cumulative doses of greater than 450 mg/m2.
Disease characteristics were: 61% poor risk for KS tumor burden, 91% poor risk for immune system, and 47% poor risk for systemic illness; 36% were poor risk for all three categories; median CD4 count 21 cells/mm3 (51% less than 50 cells/mm3); mean absolute neutrophil count at study entry approximately 3,000 cells/mm3.
Of the 693 patients with concomitant medication information, 59% were on one or more antiretroviral medications [35% zidovudine (AZT), 21% didanosine (ddI), 16% zalcitabine (ddC), and 10% stavudine (D4T)]; 85% received PCP prophylaxis (54% sulfamethoxazole/trimethoprim); 85% received antifungal medications (76% fluconazole); 72% received antivirals (56% acyclovir, 29% ganciclovir, and 16% foscarnet) and 48% patients received colony-stimulating factors (sargramostim/filgrastim) during their course of treatment.
Adverse reactions led to discontinuation of treatment in 5% of patients with AIDS-related Kaposi's sarcoma and included myelosuppression, cardiac adverse reactions, infusion-related reactions, toxoplasmosis, HFS, pneumonia, cough/dyspnea, fatigue, optic neuritis, progression of a non-KS tumor, allergy to penicillin, and unspecified reasons. Table 5 and Table 6 summarize adverse reactions reported in patients treated with doxorubicin hydrochloride liposome injection for AIDS-related Kaposi's sarcoma in a pooled analysis of the four trials.
Table 5: Hematologic Adverse Reactions Reported in Patients With AIDS-Related Kaposi's Sarcoma *This includes a subset of subjects who were retrospectively identified as having disease progression on prior systemic combination chemotherapy (at least 2 cycles of a regimen containing at least 2 of 3 treatments: bleomycin, vincristine or vinblastine, or doxorubicin) or as being intolerant to such therapy.
**This includes only subjects with AIDS-KS who had available data from the 4 pooled trials.
Patients With Refractory or Intolerant AIDS-Related Kaposi's Sarcoma
(n=74*)
Total Patients With AIDS-Related Kaposi's Sarcoma
(n=720 ** )
Neutropenia
<1,000/mm3
46%
49%
<500/mm3
11%
13%
Anemia
<10 g/dL
58%
55%
<8 g/dL
16%
18%
Thrombocytopenia
<150,000/mm3
61%
61%
<25,000/mm3
1.4%
4.2%
Table 6: Non-Hematologic Adverse Reactions Reported in ≥ 5% of Patients With AIDS-Related Kaposi's Sarcoma *This includes a subset of subjects who were retrospectively identified as having disease progression on prior systemic combination chemotherapy (at least 2 cycles of a regimen containing at least 2 of 3 treatments: bleomycin, vincristine or vinblastine, or doxorubicin) or as being intolerant to such therapy.
** This includes only subjects with AIDS-KS who had available adverse event data from the 4 pooled trials.
Adverse Reactions
Patients With Refractory or Intolerant AIDS-Related Kaposi's Sarcoma
(n=77*)
Total Patients With AIDS-Related Kaposi's Sarcoma
(n=705**)
Nausea
18%
17%
Asthenia
7%
10%
Fever
8%
9%
Alopecia
9%
9%
Alkaline Phosphatase Increase
1.3%
8%
Vomiting
8%
8%
Diarrhea
5%
8%
Stomatitis
5%
7%
Oral Moniliasis
1.3%
6%
The following additional adverse reactions were observed in 705 patients with AIDS-related Kaposi's sarcoma.
Incidence 1% to 5%
Body as a Whole: headache, back pain, infection, allergic reaction, chills.
Cardiovascular: chest pain, hypotension, tachycardia.
Cutaneous: herpes simplex, rash, itching.
Digestive: mouth ulceration, anorexia, dysphagia.
Metabolic and Nutritional: SGPT increase, weight loss, hyperbilirubinemia.
Other: dyspnea, pneumonia, dizziness, somnolence.
Incidence Less Than 1%
Body As A Whole: sepsis, moniliasis, cryptococcosis.
Cardiovascular: thrombophlebitis, cardiomyopathy, palpitation, bundle branch block, congestive heart failure, heart arrest, thrombosis, ventricular arrhythmia.
Digestive: hepatitis.
Metabolic and Nutritional Disorders: dehydration.
Respiratory: cough increase, pharyngitis.
Skin and Appendages: maculopapular rash, herpes zoster.
Special Senses: taste perversion, conjunctivitis.
Patients With Multiple Myeloma
The safety data described are from 318 patients treated with doxorubicin hydrochloride liposome injection (30 mg/m2) administered on day 4 following bortezomib (1.3 mg/m2 i.v. bolus on days 1, 4, 8 and 11) every 3 weeks, in a randomized, open-label, multicenter study (Trial 6). In this trial, patients in the doxorubicin hydrochloride liposome injection + bortezomib combination group were treated for a median number of 4.5 months (range 21 days to 13.5 months). The population was 28 to 85 years of age (median age 61), 58% male, 90% Caucasian, 6% Black, and 4% Asian and Other. Table 7 lists adverse reactions reported in 10% or more of patients treated with doxorubicin hydrochloride liposome injection in combination with bortezomib for multiple myeloma.
Table 7: Frequency of Treatment-Emergent Adverse Reactions Reported in ≥10% Patients Treated for Multiple Myeloma With Doxorubicin Hydrochloride Liposome Injection in Combination With Bortezomib 1 Peripheral neuropathy includes the following adverse reactions: peripheral sensory neuropathy, neuropathy peripheral, polyneuropathy, peripheral motor neuropathy, and neuropathy NOS.
2 Rash includes the following adverse reactions: rash, rash erythematous, rash macular, rash maculo-papular, rash pruritic, exfoliative rash, and rash generalized.
Adverse Reaction
Doxorubicin hydrochloride Liposome Injection + bortezomib
(n=318)
Bortezomib
(n=318)
Any (%)
Grade 3-4
Any (%)
Grade 3-4
Blood and lymphatic system disorders
Neutropenia
36
32
22
16
Thrombocytopenia
33
24
28
17
Anemia
25
9
21
9
General disorders and administration site conditions
Fatigue
36
7
28
3
Pyrexia
31
1
22
1
Asthenia
22
6
18
4
Gastrointestinal disorders
Nausea
48
3
40
1
Diarrhea
46
7
39
5
Vomiting
32
4
22
1
Constipation
31
1
31
1
Mucositis/Stomatitis
20
2
5
<1
Abdominal pain
11
1
8
1
Infections and infestations
Herpes zoster
11
2
9
2
Herpes simplex
10
0
6
1
Investigations
Weight decreased
12
0
4
0
Metabolism and Nutritional disorders
Anorexia
19
2
14
<1
Nervous system disorders
Peripheral Neuropathy1
42
7
45
11
Neuralgia
17
3
20
4
Paresthesia/dysesthesia
13
<1
10
0
Respiratory, thoracic and mediastinal disorders
Cough
18
0
12
0
Skin and subcutaneous tissue disorders
Rash2
22
1
18
1
Hand-foot syndrome
19
6
<1
0
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of doxorubicin hydrochloride liposome injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal and Connective Tissue Disorders: muscle spasms
Respiratory, Thoracic and Mediastinal Disorders: pulmonary embolism (in some cases fatal)
Hematologic Disorders: Secondary acute myelogenous leukemia
Skin and Subcutaneous Tissue Disorders: erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, lichenoid keratosis
Secondary Oral Neoplasms: [see Warnings and Precautions (5.4)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Based on findings in animals and its mechanism of action, doxorubicin hydrochloride liposome injection can cause fetal harm when administered to a pregnant woman; avoid the use of doxorubicin hydrochloride liposome injection during the 1st trimester. In animal reproduction studies, doxorubicin hydrochloride liposome injection was embryotoxic in rats and abortifacient in rabbits following intravenous administration during organogenesis at doses approximately 0.12 times the recommended clinical dose (see Data). Available human data do not establish the presence or absence of major birth defects and miscarriage related to the use of doxorubicin hydrochloride during the 2nd and 3rd trimesters. Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Data
Animal Data
Doxorubicin hydrochloride liposome injection was embryotoxic at doses of 1 mg/kg/day in rats and was embryotoxic and abortifacient at 0.5 mg/kg/day in rabbits (both doses are about 0.12 times the recommended dose of 50 mg/m2 human dose on a mg/m2 basis). Embryotoxicity was characterized by increased embryo-fetal deaths and reduced live litter sizes.
8.2 Lactation
It is not known whether doxorubicin hydrochloride liposome injection is present in human milk. Because many drugs, including anthracyclines, are excreted in human milk and because of the potential for serious adverse reactions in breastfed infants from doxorubicin hydrochloride liposome injection, discontinue breastfeeding during treatment with doxorubicin hydrochloride liposome injection.
8.3 Females and Males of Reproductive Potential
Verify the pregnancy status of females of reproductive potential prior to initiating doxorubicin hydrochloride liposome injection.
Contraception
Females
Doxorubicin hydrochloride liposome injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during and for 6 months after treatment with doxorubicin hydrochloride liposome injection.
Males
Doxorubicin hydrochloride liposome injection may damage spermatozoa and testicular tissue, resulting in possible genetic fetal abnormalities. Males with female sexual partners of reproductive potential should use effective contraception during and for 6 months after treatment with doxorubicin hydrochloride liposome injection [see Non-clinical Toxicology (13.1)].
Infertility
Females
In females of reproductive potential, doxorubicin hydrochloride liposome injection may cause infertility and result in amenorrhea. Premature menopause can occur with doxorubicin hydrochloride. Recovery of menses and ovulation is related to age at treatment.
Males
Doxorubicin hydrochloride liposome injection may result in oligospermia, azoospermia, and permanent loss of fertility. Sperm counts have been reported to return to normal levels in some men. This may occur several years after the end of therapy [see Non-clinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of doxorubicin hydrochloride liposome injection in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of doxorubicin hydrochloride liposome injection conducted in patients with either epithelial ovarian cancer (Trial 4) or with AIDS-related Kaposi's sarcoma (Trial 5) did not contain sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
In Trial 6, of 318 patients treated with doxorubicin hydrochloride liposome injection in combination with bortezomib for multiple myeloma, 37% were 65 years of age or older and 8% were 75 years of age or older. No overall differences in safety or efficacy were observed between these patients and younger patients.
8.6 Hepatic Impairment
The pharmacokinetics of doxorubicin hydrochloride liposome injection has not been adequately evaluated in patients with hepatic impairment. Doxorubicin is eliminated in large part by the liver. Reduce doxorubicin hydrochloride liposome injection for serum bilirubin of 1.2 mg/dL or higher.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Doxorubicin hydrochloride liposome injection is doxorubicin hydrochloride, an anthracycline topoisomerase inhibitor, that is encapsulated in pegylated liposomes for intravenous use.
The chemical name of doxorubicin hydrochloride is (8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-8-glycolyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride. The molecular formula is C27H29NO11∙HCl and the molecular weight is 579.99.
The structural formula is:
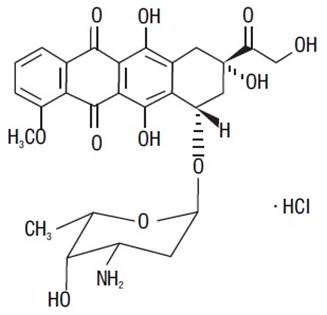
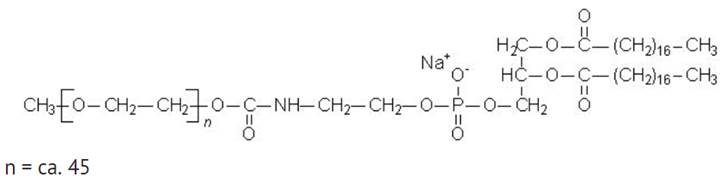
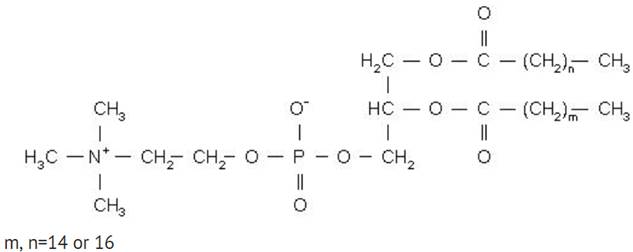
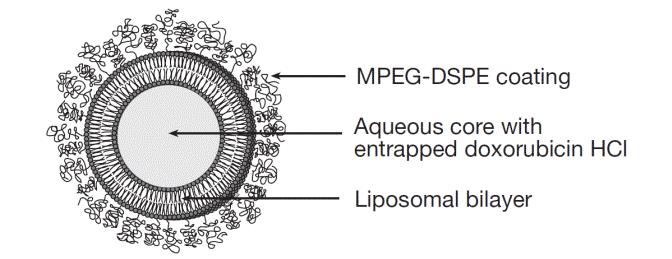
Doxorubicin hydrochloride liposome injection is a sterile, translucent, red liposomal dispersion. Each single-dose vial contains 20 mg or 50 mg doxorubicin hydrochloride at a concentration of 2 mg/mL (equivalent to 1.87 mg/mL of doxorubicin). The pegylated liposome carriers are composed of cholesterol, 3.19 mg/mL; fully hydrogenated soy phosphatidylcholine (HSPC), 9.58 mg/mL; and N-(carbonyl-methoxypolyethylene glycol 2,000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG-DSPE), 3.19 mg/mL. Each mL also contains ammonium sulfate, approximately 0.6 mg; histidine 1.50 mg as a buffer; hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment (6.0 to 7.0); and sucrose, 95.50 mg to maintain isotonicity. Greater than 90% of the drug is encapsulated in the pegylated liposomes.
MPEG-DSPE has the following structural formula:
HSPC has the following structural formula:
Representation of a pegylated liposome:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The active ingredient of doxorubicin hydrochloride liposome injection is doxorubicin hydrochloride. The mechanism of action of doxorubicin hydrochloride is thought to be related to its ability to bind DNA and inhibit nucleic acid synthesis. Cell structure studies have demonstrated rapid cell penetration and perinuclear chromatin binding, rapid inhibition of mitotic activity and nucleic acid synthesis, and induction of mutagenesis and chromosomal aberrations.
12.3 Pharmacokinetics
The pharmacokinetic parameters for total doxorubicin following a single dose of doxorubicin hydrochloride liposome injection infused over 30 minutes are presented in Table 8.
Table 8: Pharmacokinetic Parameters of Total Doxorubicin from Doxorubicin Hydrochloride Liposome Injection in Patients With AIDS-Related Kaposi's Sarcoma N=23
Mean ± Standard Error
Dose
Parameter (units)
10 mg/m2
20 mg/m2
Peak Plasma Concentration (µg/mL)
4.12 ± 0.215
8.34 ± 0.49
Plasma Clearance (L/h/m2)
0.056 ± 0.01
0.041 ± 0.004
Steady State Volume of Distribution (L/m2)
2.83 ± 0.145
2.72 ± 0.120
AUC (µg/mL∙h)
277 ± 32.9
590 ± 58.7
First Phase (λ1) Half-Life (h)
4.7 ± 1.1
5.2 ± 1.4
Second Phase (λ1) Half-Life (h)
52.3 ± 5.6
55.0 ± 4.8
Doxorubicin hydrochloride liposome injection displayed linear pharmacokinetics over the range of 10 to 20 mg/m2. Relative to doxorubicin hydrochloride liposome injection doses at or below 20 mg/m2, the pharmacokinetics of total doxorubicin following a 50 mg/m2 doxorubicin hydrochloride liposome injection dose are nonlinear. At this dose, the elimination half-life of doxorubicin hydrochloride liposome injection is longer and the clearance lower compared to a 20 mg/m2 dose.
Distribution
Direct measurement of liposomal doxorubicin shows that at least 90% of the drug (the assay used cannot quantify less than 5-10% free doxorubicin) remains liposome-encapsulated during circulation.
In contrast to doxorubicin, which displays a large volume of distribution (range 700 to 1,100 L/m2), the small steady state volume of distribution of liposomal doxorubicin suggests that doxorubicin hydrochloride liposome injection is largely confined to vascular fluid. Doxorubicin becomes available after the liposomes are extravasated. Plasma protein binding of doxorubicin hydrochloride liposome injection has not been determined; the plasma protein binding of doxorubicin is approximately 70%.
Metabolism
Doxorubicinol, the major metabolite of doxorubicin, was detected at concentrations of 0.8 to 26.2 ng/mL in the plasma of patients who received 10 or 20 mg/m2 doxorubicin hydrochloride liposome injection.
Elimination
The plasma clearance of total doxorubicin from doxorubicin hydrochloride liposome injection was 0.041 L/h/m2 at a dose of 20 mg/m2. Following administration of doxorubicin hydrochloride, the plasma clearance of doxorubicin is 24 to 35 L/h/m2.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Mutagenicity or carcinogenicity studies have not been conducted with doxorubicin hydrochloride liposome injection, however doxorubicin was shown to be mutagenic in the in vitro Ames assay, and clastogenic in multiple in vitro assays (CHO cell, V79 hamster cell, human lymphoblast, and SCE assays) and the in vivo mouse micronucleus assay. The possible adverse effects on fertility in animals have not been adequately evaluated. Doxorubicin hydrochloride liposome injection resulted in mild to moderate ovarian and testicular atrophy in mice after administration of a single dose of 36 mg/kg (about 2 times the 50 mg/m2 human dose on a mg/m2 basis). Decreased testicular weights and hypospermia were observed in rats after repeat doses ≥ 0.25 mg/kg/day (about 0.03 times the 50 mg/m2 human dose on a mg/m2 basis), and diffuse degeneration of the seminiferous tubules and a marked decrease in spermatogenesis were observed in dogs after repeat doses of 1 mg/kg/day (about 0.4 times the 50 mg/m2 human dose on a mg/m2 basis).
-
14 CLINICAL STUDIES
14.1 Ovarian Cancer
Doxorubicin hydrochloride liposome injection was studied in three open-label, single-arm, clinical studies of 176 patients with metastatic ovarian cancer (Trials 1, 2, and 3). One hundred forty-five of these patients were refractory to both paclitaxel- and platinum-based chemotherapy regimens, defined as disease progression while on treatment or relapse within 6 months of completing treatment. Patients received doxorubicin hydrochloride liposome injection at 50 mg/m2 every 3 or 4 weeks for 3-6+ cycles in the absence of dose-limiting toxicity or disease progression.
The median age at diagnosis ranged from 52 to 64 years in the 3 studies, and the range was 22 to 85. Most patients had International Federation of Obstetricians and Gynecologists (FIGO) stage III or IV disease (ranging from 83% to 93%). Approximately one third of the patients had three or more prior lines of therapy (ranging from 22% to 33%).
The primary outcome measure was confirmed response rate based on Southwestern Oncology Group (SWOG) criteria for patients refractory to both paclitaxel- and a platinum-containing regimen. Secondary efficacy parameters were time to response, duration of response, and time to progression.
The response rates for the individual single arm trials are given in Table 9 below.
Table 9: Response Rates in Patients With Refractory Ovarian Cancer From Single Arm Ovarian Cancer Trials Trial 1 (U.S.)
N=27
Trial 2 (U.S.)
N=82
Trial 3 (non-U.S.)
N=36
Response Rate
22.2%
17.1%
0%
95% Confidence Interval
8.6% - 42.3%
9.7% - 27.0%
0.0% - 9.7%
In a pooled analysis of Trials 1-3, the response rate for all patients refractory to paclitaxel and platinum agents was 13.8% (95% CI 8.1% to 19.3%). The median time to progression was 15.9 weeks, the median time to response was 17.6 weeks, and the duration of response was 39.4 weeks.
In Trial 4, a randomized, multicenter, open-label, trial in 474 patients with epithelial ovarian cancer after platinum-based chemotherapy, patients were randomized to receive either doxorubicin hydrochloride liposome injection 50 mg/m2 every 4 weeks (n=239) or topotecan 1.5 mg/m2 daily for 5 consecutive days every 3 weeks (n=235). Patients were stratified according to platinum sensitivity (response to initial platinum-based therapy and a progression-free interval of greater than 6 months off treatment) and the presence of bulky disease (tumor mass greater than 5 cm in size). The primary outcome measure was time to progression (TTP). Other endpoints included overall survival and objective response rate.
Of the 474 patients, the median age at diagnosis was 60 years (range 25 to 87), 90% were FIGO stage III and IV; 46% were platinum sensitive; and 45% had bulky disease.
There was no statistically significant difference in TTP between the two arms. Results are provided in Table 10.
Table 10: Results of Efficacy Analyses1 1Analysis based on investigators' strata for protocol defined ITT population.
2Kaplan-Meier estimates.
3p-value is based on the stratified log-rank test.
4Hazard ratio is based on Cox proportional-hazard model with the treatment as single independent variable. A hazard ratio less than 1 indicates an advantage for doxorubicin hydrochloride liposome injection.
5p-value not adjusted for multiple comparisons.
Protocol Defined ITT Population
Doxorubicin Hydrochloride Liposome Injection
(n=239)
Topotecan
(n=235)
TTP (Protocol Specified Primary Endpoint)
Median (Months)2
4.1
4.2
p-value3
0.62
Hazard Ratio4
0.96
95% CI for Hazard Ratio
(0.76, 1.20)
Overall Survival
Median (Months)2
14.4
13.7
p-value5
0.05
Hazard Ratio4
0.82
95% CI for Hazard Ratio
(0.68, 1.00)
Response Rate
Overall Response n (%)
47 (19.7)
40 (17.0)
Complete Response n (%)
9 (3.8)
11 (4.7)
Partial Response n (%)
38 (15.9)
29 (12.3)
Median Duration of Response (Months)2
6.9
5.9
14.2 AIDS-Related Kaposi's Sarcoma
Doxorubicin hydrochloride liposome injection was studied in an open-label, single-arm, multicenter study at a dose of 20 mg/m2 every 3 weeks, until disease progression or unacceptable toxicity (Trial 5).
Data is described for a cohort of 77 patients retrospectively identified as having disease progression on prior systemic combination chemotherapy (at least two cycles of a regimen containing at least two of three treatments: bleomycin, vincristine or vinblastine, or doxorubicin) or as being intolerant to such therapy. Forty-nine of the 77 (64%) patients had received prior doxorubicin hydrochloride.
The median time on study was 5.1 months (range 1 day to 15 months). The median cumulative dose of doxorubicin hydrochloride liposome injection was 154 mg/m2 (range 20 to 620 mg/m2). Among the 77 patients, mean age was 38 years (range 24 to 54); 87% were Caucasian, 5% Hispanic, 4% Black, and 4% Asian/Other/Unknown; median CD4 count was 10 cells/mm3; ACTG staging criteria were 78% poor risk for tumor burden, 96% poor risk for immune system, and 58% poor risk for systemic illness at baseline; and mean Karnofsky status score was 74%. All patients had cutaneous or subcutaneous lesions, 40% also had oral lesions, 26% pulmonary lesions, and 14% had lesions of the stomach/intestine.
Two analyses of tumor response were used: one based on investigator assessment of changes in lesions based on modified ACTG criteria (partial response defined as no new lesions, sites of disease, or worsening edema; flattening of ≥50% of previously raised lesions or area of indicator lesions decreasing by ≥50%; and response lasting at least 21 days with no prior progression), and one based on changes in up to five prospectively indentified representative indicator lesions (partial response defined as flattening of ≥50% of previously raised indicator lesions, or >50% decrease in the area of indicator lesions and lasting at least 21 days with no prior progression).
Of the 77 patients, 34 were evaluable for investigator assessment and 42 were evaluable for indicator lesion assessment; analyses of tumor responses are shown in Table 11.
Table 11: Response in Patients with Refractory1 AIDS-Related Kaposi's Sarcoma 1 Patients with disease that progressed on prior combination chemotherapy or who were intolerant to such therapy.
2 There were no complete responses in this population.
Investigator Assessment
All Evaluable Patients
(n=34)
Evaluable Patients Who Received Prior Doxorubicin
(n=20)
Response2
Partial (PR)
27%
30%
Stable
29%
40%
Progression
44%
30%
Duration of PR (Days)
Median
73
89
Range
42+ to 210+
42+ to 210+
Time to PR (Days)
Median
43
53
Range
15 to 133
15 to 109
Indicator Lesion Assessment
All Evaluable Patients
(n=42)
Evaluable Patients Who Received Prior Doxorubicin
(n=23)
Response2
Partial (PR)
48%
52%
Stable
26%
30%
Progression
26%
17%
Duration of PR (Days)
Median
71
79
Range
22+ to 210+
35 to 210+
Time to PR (Days)
Median
22
48
Range
15 to 109
15 to 109
Retrospective efficacy analyses were performed in two trials that had subsets of patients who received single-agent doxorubicin hydrochloride liposome injection and who were on stable antiretroviral therapy for at least 60 days prior to enrollment and until a response was demonstrated. In one trial, 7 of 17 (40%) patients had a durable response (median duration not reached but was longer than 11.6 months). In the second trial, 4 of 11 patients (40%) on a stable antiretroviral therapy demonstrated durable responses.
14.3 Multiple Myeloma
The efficacy of doxorubicin hydrochloride liposome injection in combination with bortezomib was evaluated in Trial 6, a randomized, open-label, international, multicenter study in 646 patients who had not previously received bortezomib and whose disease progressed during or after at least one prior therapy. Patients were randomized (1:1) to receive either doxorubicin hydrochloride liposome injection (30 mg/m2) administered intravenous on day 4 following bortezomib (1.3 mg/m2 intravenous on days 1, 4, 8 and 11) or bortezomib alone every 3 weeks for up to 8 cycles or until disease progression or unacceptable toxicity. Patients who maintained a response were allowed to receive further treatment. The median number of cycles in each treatment arm was 5 (range 1-18).
The baseline demographics and clinical characteristics of the patients with multiple myeloma were similar between treatment arms (Table 12).
Table 12: Summary of Baseline Patient and Disease Characteristics Patient Characteristics
Doxorubicin Hydrochloride Liposome Injection + bortezomib
n=324
bortezomib
n=322
Median age in years (range)
61 (28, 85)
62 (34, 88)
% Male/female
58 / 42
54 / 46
% Caucasian/Black/other
90 / 6 / 4
94 / 4 / 2
Disease Characteristics
% with IgG/IgA/Light chain
57 / 27 / 12
62 / 24 / 11
% β2-microglobulin group
≤2.5 mg/L
14
14
>2.5 mg/L and ≤5.5 mg/L
56
55
>5.5 mg/L
30
31
Serum M-protein (g/dL): Median (Range)
2.5 (0-10.0)
2.7 (0-10.0)
Urine M-protein (mg/24 hours): Median (Range)
107 (0-24883)
66 (0-39657)
Median Months Since Diagnosis
35.2
37.5
% Prior Therapy
One
34
34
More than one
66
66
Prior Systemic Therapies for Multiple Myeloma
Corticosteroid (%)
99
>99
Anthracyclines
68
67
Alkylating agent (%)
92
90
Thalidomide/lenalidomide (%)
40
43
Stem cell transplantation (%)
57
54
The primary outcome measure was time to progression (TTP). TTP was defined as the time from randomization to the first occurrence of progressive disease or death due to progressive disease. The combination arm demonstrated significant improvement in TTP. As the prespecified primary objective was achieved at the interim analysis, patients in the bortezomib monotherapy group were then allowed to receive the doxorubicin hydrochloride liposome injection + bortezomib combination. Efficacy results are as shown in Table 13 and Figure 1.
Table 13: Efficacy of Doxorubicin Hydrochloride Liposome Injection in Combination With Bortezomib in the Treatment of Patients With Multiple Myeloma 1 Kaplan Meier estimate.
2 Hazard ratio based on stratified Cox proportional hazards regression. A hazard ratio <1 indicates an advantage for doxorubicin hydrochloride liposome injection + bortezomib.
3 Stratified log-rank test.
4 RR as per EBMT criteria.
5 Cochran-Mantel-Haenszel test adjusted for the stratification factors.
Endpoint
Doxorubicin Hydrochloride Liposome Injection + bortezomib
n=324
Bortezomib
n=322
Time to Progression1
Progression or death due to progression (n)
99
150
Censored (n)
225
172
Median in days (months)
282 (9.3)
197 (6.5)
95% CI
250; 338
170; 217
Hazard ratio2
0.55
(95% CI)
(0.43, 0.71)
p-value3
<0.001
Response (n)4
303
310
% Complete Response (CR)
5
3
% Partial Response (PR)
43
40
% CR + PR
48
43
p-value5
0.25
Median Duration of Response (months)
10.2
7.0
(95% CI)
(10.2; 12.9)
(5.9; 8.3)
Figure 1- Time to Progression Kaplan-Meier Curve
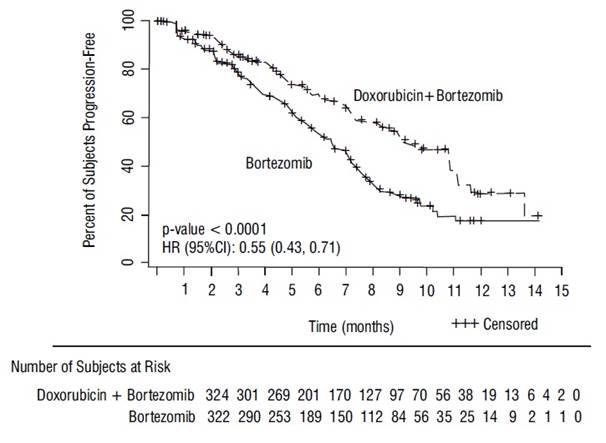
At the final analysis of survival, 78% of subjects in the doxorubicin hydrochloride liposome injection and bortezomib combination therapy group and 80% of subjects in the bortezomib monotherapy group had died after a median follow up of 8.6 years. The median survival was 33 months in the doxorubicin hydrochloride liposome injection and bortezomib combination therapy group and 31 months in the bortezomib monotherapy group. There was no difference observed in overall survival at the final analysis [HR for doxorubicin hydrochloride liposome injection + bortezomib vs. bortezomib = 0.96 (95% CI 0.80, 1.14)].
Seventy-eight percent of subjects in the doxorubicin hydrochloride liposome injection and bortezomib combination therapy group and 80% of subjects in the bortezomib monotherapy group had received subsequent therapy.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Doxorubicin hydrochloride liposome injection is a sterile, translucent, red liposomal dispersion in 10 mL or 30 mL glass, single dose vials.
The following individually cartoned vials are available:
Table 14 Vial Concentration
Vial Size
NDC number
20 mg/10 mL
(2 mg/ mL)
10 mL
70710-1530-1
50 mg/25 mL
(2 mg/mL)
30 mL
70710-1531-1
Refrigerate unopened vials of doxorubicin hydrochloride liposome injection at 2° to 8°C (36° to 46°F). Do not freeze. Discard unused portion.
Doxorubicin hydrochloride liposome injection is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise patients to contact their healthcare provider if they develop symptoms of heart failure [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients about the symptoms of infusion related reactions and to seek immediate medical attention if they develop any of these symptoms [see Warnings and Precautions (5.2)].
Myelosuppression
Advise patients to contact their healthcare provider for a new onset fever or symptoms of infection.
Hand-Foot Syndrome
Advise patients to notify their healthcare provider if they experience tingling or burning, redness, flaking, bothersome swelling, small blisters, or small sores on the palms of their hands or soles of their feet (symptoms of Hand-Foot Syndrome) [see Warnings and Precautions (5.3)].
Stomatitis
Advise patients to notify their healthcare provider if they develop painful redness, swelling, or sores in the mouth (symptoms of stomatitis).
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
Advise females and males of reproductive potential to use effective contraception during and for 6 months following treatment with doxorubicin hydrochloride liposome injection [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with doxorubicin hydrochloride liposome injection [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential that doxorubicin hydrochloride liposome injection may cause temporary or permanent infertility [see Use in Specific Populations (8.3)].
Discoloration of Urine and Body Fluids
Inform patients that following doxorubicin hydrochloride liposome injection administration, a reddish-orange color to the urine and other body fluids may be observed. This nontoxic reaction is due to the color of the product and will dissipate as the drug is eliminated from the body.
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel – 20 mg/10 mL Container Label
NDC: 70710-1530-1
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
10 mL Single-Dose Vial
Rx only
Principal Display Panel – 20 mg/10 mL Carton Label
NDC: 70710-1530-1
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
10 mL Single-Dose Vial
Rx only
Principal Display Panel – 50 mg/25 mL Container Label
NDC: 70710-1531-1
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
25 mL Single-Dose Vial
Rx only

Principal Display Panel – 50 mg/25 mL Carton Label
NDC: 70710-1531-1
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL
(2 mg/mL)
Cytotoxic Agent Must be diluted
LIPOSOMAL FORMULATION – DO NOT SUBSTITUTE FOR DOXORUBICIN HCL
FOR INTRAVENOUS INFUSION ONLY
Sterile
Refrigerate at 2⁰ to 8℃ (36⁰ to 46℉)
Do not freeze
25 mL Single-Dose Vial
Rx only
-
INGREDIENTS AND APPEARANCE
DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injectable, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-1530 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength N-(CARBONYL-METHOXYPOLYETHYLENE GLYCOL 2000)-1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE (UNII: IC5THO9L3U) 3.19 mg in 1 mL HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) 9.58 mg in 1 mL CHOLESTEROL (UNII: 97C5T2UQ7J) 3.19 mg in 1 mL AMMONIUM SULFATE (UNII: SU46BAM238) 0.6 mg in 1 mL SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-1530-1 1 in 1 CARTON 09/14/2020 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212299 09/14/2020 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injectable, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-1531 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength N-(CARBONYL-METHOXYPOLYETHYLENE GLYCOL 2000)-1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE (UNII: IC5THO9L3U) 3.19 mg in 1 mL HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) 9.58 mg in 1 mL CHOLESTEROL (UNII: 97C5T2UQ7J) 3.19 mg in 1 mL AMMONIUM SULFATE (UNII: SU46BAM238) 0.6 mg in 1 mL SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-1531-1 1 in 1 CARTON 09/14/2020 1 25 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212299 09/14/2020 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Registrant - Zydus Lifesciences Global FZE (850107010) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650348852 ANALYSIS(70710-1530, 70710-1531) , MANUFACTURE(70710-1530, 70710-1531)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.