PACLITAXEL injection, powder, lyophilized, for suspension
Paclitaxel by
Drug Labeling and Warnings
Paclitaxel by is a Prescription medication manufactured, distributed, or labeled by Sandoz Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PACLITAXEL PROTEIN-BOUND PARTICLES FOR INJECTABLE SUSPENSION (ALBUMIN-BOUND) safely and effectively. See full prescribing information for PACLITAXEL PROTEIN-BOUND PARTICLES FOR INJECTABLE SUSPENSION (ALBUMIN-BOUND).
PACLITAXEL protein-bound particles for injectable suspension (albumin-bound), for intravenous use
Initial U.S. Approval: 2005WARNING: SEVERE MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
- Do not administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) therapy to patients with baseline neutrophil counts of less than 1,500 cells/mm 3. ( 4)
- Monitor for neutropenia, which may be severe and result in infection or sepsis. ( 5.1, 5.3)
- Perform frequent complete blood cell counts on all patients receiving paclitaxel protein-bound particles for injectable suspension (albumin-bound). ( 5.1, 5.3)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE(1.2, 1.3) 02/2025 DOSAGE AND ADMINISTRATION(2.3, 2.4, 2.5, 2.6) 02/2025 WARNINGS AND PRECAUTIONS(5.1, 5.2, 5.6) 02/2025
INDICATIONS AND USAGE
- Metastatic breast cancer, after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated. (1.1)
- Locally advanced or metastatic non-small cell lung cancer (NSCLC), as first-line treatment in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy. (1.2)
- Metastatic adenocarcinoma of the pancreas as first-line treatment, in combination with gemcitabine. (1.3)
DOSAGE AND ADMINISTRATION
- Do not substitute paclitaxel protein-bound particles for injectable suspension (albumin-bound) for other paclitaxel products. (2.1)
- Extravasation: Closely monitor the infusion site for extravasation and infiltration. (2.1)
- Metastatic Breast Cancer (MBC): Recommended dosage of paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 260 mg/m2 intravenously over 30 minutes every 3 weeks. (2.2)
- Non-Small Cell Lung Cancer (NSCLC): Recommended dosage of paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 100 mg/m2 intravenously over 30 minutes on Days 1, 8, and 15 of each 21-day cycle; administer carboplatin on Day 1 of each 21-day cycle immediately after paclitaxel protein-bound particles for injectable suspension (albumin-bound). (2.2)
- Adenocarcinoma of the Pancreas: Recommended dosage of paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 125 mg/m2 intravenously over 30-40 minutes on Days 1, 8, and 15 of each 28-day cycle; administer gemcitabine on Days 1, 8, and 15 of each 28-day cycle immediately after paclitaxel protein-bound particles for injectable suspension (albumin-bound). (2.4)
- Use in Patients with Hepatic Impairment: Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is not recommended for use in patients with AST > 10 x ULN; or bilirubin > 5 x ULN or with metastatic adenocarcinoma of the pancreas who have moderate to severe hepatic impairment. For MBC or NSCLC, reduce starting dose in patients with moderate to severe hepatic impairment. (2.5)
- Dose Reductions for Adverse Reactions: Dose reductions or discontinuation may be needed based on severe hematologic, neurologic, cutaneous, or gastrointestinal toxicities. (2.6)
DOSAGE FORMS AND STRENGTHS
For injectable suspension: white to yellow, sterile, lyophilized powder containing 100 mg of paclitaxel formulated as albumin-bound particles in single-dose vial for reconstitution. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Sensory neuropathy occurs frequently and may require dose reduction or treatment interruption. (5.2)
- Sepsis occurred in patients with or without neutropenia who received paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine; interrupt paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine until sepsis resolves, and if neutropenia, until neutrophils are at least 1500 cells/mm3, then resume treatment at reduced dose levels. (5.3)
- Pneumonitis occurred with the use of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine; permanently discontinue treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine. (5.4)
- Severe hypersensitivity reactions with fatal outcome have been reported. Do not rechallenge with this drug. (4, 5.5)
- Exposure and toxicity of paclitaxel can be increased in patients with hepatic impairment, consider dose reduction and closely monitor patients with hepatic impairment. (2.5, 5.6)
- Paclitaxel protein-bound particles for injectable suspension (albumin-bound)contains albumin derived from human blood, which has a theoretical risk of viral transmission. (5.7)
- Paclitaxel protein-bound particles for injectable suspension (albumin-bound)can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.8, 8.1, 8.3)
ADVERSE REACTIONS
- The most common adverse reactions (≥ 20%) in metastatic breast cancer are alopecia, neutropenia, sensory neuropathy, abnormal ECG, fatigue/asthenia, myalgia/arthralgia, AST elevation, alkaline phosphatase elevation, anemia, nausea, infections, and diarrhea. ( 6.1)
- The most common adverse reactions (≥ 20%) in NSCLC are anemia, neutropenia, thrombocytopenia, alopecia, peripheral neuropathy, nausea, and fatigue. (6.1)
- The most common (≥ 20%) adverse reactions of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in adenocarcinoma of the pancreas are neutropenia, fatigue, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, pyrexia, vomiting, decreased appetite, rash, and dehydration. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (7)
DRUG INTERACTIONS
Use caution when concomitantly administering paclitaxel protein-bound particles for injectable suspension (albumin-bound) with inhibitors or inducers of either CYP2C8 or CYP3A4. ( 7)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NEUTROPENIA
1 INDICATIONS AND USAGE
1.1 Metastatic Breast Cancer
1.2 Non-Small Cell Lung Cancer
1.3 Adenocarcinoma of the Pancreas
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage for Metastatic Breast Cancer
2.3 Recommended Dosage for Non-Small Cell Lung Cancer
2.4 Recommended Dosage for Adenocarcinoma of the Pancreas
2.5 Dosage Modifications for Hepatic Impairment
2.6 Dosage Modifications for Adverse Reactions
2.7 Preparation for Intravenous Administration
2.8 Stability
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Myelosuppression
5.2 Severe Neuropathy
5.3 Sepsis
5.4 Pneumonitis
5.5 Severe Hypersensitivity
5.6 Use in Patients with Hepatic Impairment
5.7 Albumin (Human)
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Metastatic Breast Cancer
14.2 Non-Small Cell Lung Cancer
14.3 Adenocarcinoma of the Pancreas
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: NEUTROPENIA
- Do not administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) therapy to patients who have baseline neutrophil counts of less than 1,500 cells/mm 3[see Contraindications (4)].
- Monitor for neutropenia, which may be severe and result in infection or sepsis, it is recommended that frequent [see Warnings and Precautions (5.1, 5.3)].
- Perform frequent complete blood cell counts on all patients receiving paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Contraindications (4), Warnings and Precautions (5.1, 5.3)].
-
1 INDICATIONS AND USAGE
1.1 Metastatic Breast Cancer
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
1.2 Non-Small Cell Lung Cancer
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is indicated for the first-line treatment of locally advanced or metastatic non-small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
DO NOT SUBSTITUTE FOR OR WITH OTHER PACLITAXEL FORMULATIONS. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) has different dosage and administration instructions from other paclitaxel products.
Closely monitor the infusion site for extravasation or drug infiltration during administration. Limiting the infusion of paclitaxel protein-bound particles for injectable suspension (albumin-bound) to 30 minutes may reduce the risk of infusion-related reactions [see Adverse Reactions (6.2)] .
Consider premedication in patients who have had prior hypersensitivity reactions to paclitaxel protein-bound particles for injectable suspension (albumin-bound). Do not re-challenge patients who experience a severe hypersensitivity reaction to paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Contraindications (4)and Warnings and Precautions (5.5)] .
2.2 Recommended Dosage for Metastatic Breast Cancer
After failure of combination chemotherapy for metastatic breast cancer or relapse within 6 months of adjuvant chemotherapy, the recommended regimen for paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 260 mg/m 2administered intravenously over 30 minutes every 3 weeks.
2.3 Recommended Dosage for Non-Small Cell Lung Cancer
The recommended dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 100 mg/m2 administered as an intravenous infusion over 30 minutes on Days 1, 8, and 15 of each 21-day cycle. Administer carboplatin on Day 1 of each 21-day cycle immediately after paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see 14.2].
2.4 Recommended Dosage for Adenocarcinoma of the Pancreas
The recommended dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) is 125 mg/m2 administered as an intravenous infusion over 30-40 minutes on Days 1, 8, and 15 of each 28-day cycle. Administer gemcitabine immediately after paclitaxel protein-bound particles for injectable suspension (albumin-bound) on Days 1, 8, and 15 of each 28-day cycle [see Clinical Studies (14.3)].
2.5 Dosage Modifications for Hepatic Impairment
For patients with moderate or severe hepatic impairment, reduce the starting dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) as shown in Table 1.
Table 1: Recommendations for Starting Dose in Patients with Moderate and Severe Hepatic Impairment AST = Aspartate Aminotransferase; MBC = Metastatic Breast Cancer; NSCLC = Non-Small Cell Lung Cancer; ULN = Upper limit of normal. a. Dosage recommendations are for the first course of therapy. The need for further dose adjustments in subsequent courses should be based on individual tolerance. b. A dose increase to 260 mg/m 2 for patients with metastatic breast cancer or 100 mg/m 2 for patients with non small cell lung cancer in subsequent courses should be considered if the patient tolerates the reduced dose for two cycles. c. Patients with bilirubin levels above the upper limit of normal were excluded from clinical trials for pancreatic or lung cancer. AST Levels Bilirubin Levels Paclitaxel Protein-Bound Particles for Injectable Suspension (Albumin-Bound) Dose a MBC NSCLC c Adenocarcinoma of Pancreas c Moderate < 10 x ULN AND > 1.5 to ≤ 3 x ULN 200 mg/m 2b 80 mg/m 2b not recommended Severe < 10 x ULN AND > 3 to ≤ 5 x ULN 200 mg/m 2b 80 mg/m 2b not recommended > 10 x ULN OR > 5 x ULN not recommended not recommended not recommended 2.6 Dosage Modifications for Adverse Reactions
Metastatic Breast Cancer
Patients who experience severe neutropenia (neutrophils less than 500 cells/mm 3 for a week or longer) or severe sensory neuropathy during paclitaxel protein-bound particles for injectable suspension (albumin-bound) therapy should have dosage reduced to 220 mg/m 2for subsequent courses of paclitaxel protein-bound particles for injectable suspension (albumin-bound). For recurrence of severe neutropenia or severe sensory neuropathy, additional dose reduction should be made to 180 mg/m 2. For Grade 3 sensory neuropathy hold treatment until resolution to Grade 1 or 2, followed by a dose reduction for all subsequent courses of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Contraindications (4), Warnings and Precautions (5.1, 5.2)and Adverse Reactions (6.1)].
Non-Small Cell Lung Cancer
- Do not administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) on Day 1 of a cycle until absolute neutrophil count (ANC) is at least 1500 cells/mm 3 and platelet count is at least 100,000 cells/mm 3 [see Contraindications(4), Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- In patients who develop severe neutropenia or thrombocytopenia withhold treatment until counts recover to an absolute neutrophil count of at least 1500 cells/mm 3 and platelet count of at least 100,000 cells/mm 3 on Day 1 or to an absolute neutrophil count of at least 500 cells/mm 3 and platelet count of at least 50,000 cells/mm 3 on Days 8 or 15 of the cycle. Upon resumption of dosing, permanently reduce paclitaxel protein-bound particles for injectable suspension (albumin-bound) and carboplatin doses as outlined in Table 2.
- Withhold paclitaxel protein-bound particles for injectable suspension (albumin-bound) for Grade 3-4 peripheral neuropathy. Resume paclitaxel protein-bound particles for injectable suspension (albumin-bound) and carboplatin at reduced doses (see Table 2) when peripheral neuropathy improves to Grade 1 or completely resolves [see Warnings and Precautions(5.2) and Adverse Reactions (6.1)] .
Table 2: Permanent Dose Reductions for Hematologic and Neurologic Adverse Reactions in NSCLC Adverse Reaction Occurrence Weekly
paclitaxel protein-bound particles for injectable suspension (albumin-bound) Dose
(mg/m 2)Every 3-Week Carboplatin Dose
(AUC mg·min/mL)Neutropenic Fever (ANC less than 500/mm 3 with fever >38°C)
OR
First 75 4.5 Delay of next cycle by more than 7 days for ANC less than 1500/mm 3
OR
Second 50 3 ANC less than 500/mm 3 for more than 7 days Third Discontinue Treatment
Platelet count less than 50,000/mm 3 First 75 4.5 Second Discontinue Treatment
Severe sensory Neuropathy – Grade 3 or 4 First 75 4.5 Second 50 3 Third Discontinue Treatment
Adenocarcinoma of the Pancreas
Dose level reductions for patients with adenocarcinoma of the pancreas, as referenced in Tables 4 and 5, are provided in Table 3
Table 3: Dose Level Reductions for Patients with Adenocarcinoma of the Pancreas Dose Level
Paclitaxel protein-bound particles for injectable suspension
(albumin-bound) (mg/m 2)
Gemcitabine (mg/m 2)
Full dose
125
1000
1 st dose reduction
100 800 2 nd dose reduction
75 600 If additional dose reduction required
Discontinue Discontinue
Recommended dose modifications for neutropenia and thrombocytopenia for patients with adenocarcinoma of the pancreas are provided in Table 4.
Table 4: Dose Recommendation and Modifications for Neutropenia and/or Thrombocytopenia at the Start of a Cycle orwithin a Cycle for Patients with Adenocarcinoma of the Pancreas Cycle Day
ANC (cells/mm 3)
Platelet count (cells/mm 3)
Paclitaxel protein-bound particles for injectable suspension
(albumin-bound) / Gemcitabine
Day 1
< 1500
OR < 100,000
Delay doses until recovery
Day 8
500 to < 1000
OR 50,000 to < 75,000
Reduce 1 dose level
< 500
OR < 50,000
Withhold doses
Day 15: If Day 8 doses were reduced or given without modification:
500 to < 1000
OR 50,000 to < 75,000
Reduce 1 dose level from Day 8
< 500
OR < 50,000
Withhold doses
Day 15: If Day 8 doses were withheld:
≥ 1000
OR ≥ 75,000
Reduce 1 dose level from Day 1
500 to < 1000
OR 50,000 to < 75,000
Reduce 2 dose levels from Day 1
< 500
OR < 50,000
Withhold doses
ANC = Absolute Neutrophil Count
Recommended dose modifications for other adverse reactions in patients with adenocarcinoma of the pancreas are provided in Table 5.Table 5: Dose Modifications for Other Adverse Reactions in Patients with Adenocarcinoma of the Pancreas Adverse Reaction Paclitaxel protein-bound particles for injectable suspension (albumin-bound) Gemcitabine Febrile Neutropenia:
Grade 3 or 4Withhold until fever resolves and ANC ≥ 1500; resume at next lower dose level Peripheral Neuropathy:
Grade 3 or 4Withhold until improves to ≤ Grade 1; resume at next lower dose level No dose reduction Cutaneous Toxicity:
Grade 2 or 3Reduce to next lower dose level; discontinue treatment if toxicity persists Gastrointestinal Toxicity:
Grade 3 mucositis or diarrheaWithhold until improves to ≤ Grade 1; resume at next lower dose level 2.7 Preparation for Intravenous Administration
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1The use of gloves is recommended. If paclitaxel protein-bound particles for injectable suspension (albumin-bound) (lyophilized cake or reconstituted suspension) contacts the skin, wash the skin immediately and thoroughly with soap and water. Following topical exposure to paclitaxel, events may include tingling, burning, and redness. If paclitaxel protein-bound particles for injectable suspension (albumin-bound) contacts mucous membranes, the membranes should be flushed thoroughly with water.
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is supplied as a sterile lyophilized powder for reconstitution before use.
Read the entire preparation instructions prior to reconstitution.
- Aseptically, reconstitute each vial by injecting 20 mL of 0.9% Sodium Chloride Injection, USP.
- Slowly inject the 20 mL of 0.9% Sodium Chloride Injection, USP, over a minimum of 1 minute, using the
sterile syringe to direct the solution flow onto the INSIDE WALL OF THE VIAL.
- DO NOT INJECT the 0.9% Sodium Chloride Injection, USP, directly onto the lyophilized cake as this
will result in foaming. - Once the injection is complete, allow the vial to sit for a minimum of 5 minutes to ensure proper wetting of
the lyophilized cake/powder. - Gently swirl and/or invert the vial slowly for at least 2 minutes until complete dissolution of any cake/powder
occurs. Avoid generation of foam. - If foaming or clumping occurs, stand solution for at least 15 minutes until foam subsides.
Each mL of the reconstituted formulation will contain 5 mg/mL paclitaxel.
The reconstituted suspension should be milky and homogenous without visible particulates. If particulates or settling are visible, the vial should be gently inverted again to ensure complete resuspension prior to use. Discard the reconstituted suspension if precipitates are observed. Discard any unused portion.
Calculate the exact total dosing volume of 5 mg/mL suspension required for the patient and slowly withdraw the dosing volume of the reconstituted suspension from the vial(s) into a syringe: Dosing volume (mL)=Total dose (mg)/5 (mg/mL).
Inject the appropriate amount of reconstituted paclitaxel protein-bound particles for injectable suspension (albumin-bound) into an empty, sterile intravenous bag [plasticized polyvinyl chloride (PVC) containers, PVC or non-PVC type intravenous bag]. The use of specialized DEHP-free solution containers or administration sets is not necessary to prepare or administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) infusions. The use of medical devices containing silicone oil as a lubricant (i.e., syringes and intravenous bags) to reconstitute and administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) may result in the formation of proteinaceous strands.
Visually inspect the reconstituted paclitaxel protein-bound particles for injectable suspension (albumin-bound) suspension in the intravenous bag prior to administration. Discard the reconstituted suspension if proteinaceous strands, particulate matter, or discoloration are observed.
2.8 Stability
Unopened vials of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are stable until the date indicated on the package when stored between 20°C to 25°C (68°F to 77°F) in the original package. Neither freezing nor refrigeration adversely affects the stability of the product.
Stability of Reconstituted Suspension in the Vial
Reconstituted paclitaxel protein-bound particles for injectable suspension (albumin-bound) in the vial should be used immediately, but may be refrigerated at 2°C to 8°C (36°F to 46°F) for a maximum of 24 hours if necessary. If not used immediately, each vial of reconstituted suspension should be replaced in the original carton to protect it from bright light. Discard any unused portion.Stability of Reconstituted Suspension in the Infusion Bag
The suspension for infusion when prepared as recommended in an infusion bag should be used immediately, but may be refrigerated at 2°C to 8°C (36°F to 46°F) and protected from bright light for a maximum of 24 hours.The total combined refrigerated storage time of reconstituted paclitaxel protein-bound particles for injectable suspension (albumin-bound) in the vial and in the infusion bag is 24 hours. This may be followed by storage in the infusion bag at ambient temperature (approximately 25°C) and lighting conditions for a maximum of 4 hours.
Discard any unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is contraindicated in patients with:
- Baseline neutrophil counts of < 1,500 cells/mm 3[see Warnings and Precautions (5.1)]
- A history of severe hypersensitivity reactions to paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Warnings and Precautions (5.5)]
-
5 WARNINGS AND PRECAUTIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Myelosuppression
Severe myelosuppression (primarily neutropenia) is dose-dependent and a dose-limiting toxicity of paclitaxel protein-bound particles for injectable suspension (albumin-bound). In clinical studies, Grade 3-4 neutropenia occurred in 34% of patients with metastatic breast cancer (MBC), 47% of patients with non-small cell lung cancer (NSCLC), and 38% of patients with pancreatic cancer.
Monitor for severe neutropenia and thrombocytopenia by performing complete blood cell counts frequently, including prior to dosing on Day 1 (for MBC) and Days 1, 8, and 15 (for NSCLC and for pancreatic cancer). Do not administer paclitaxel protein-bound particles for injectable suspension (albumin-bound) to patients with baseline absolute neutrophil counts (ANC) of less than 1,500 cells/mm 3[see Contraindications (4)] .
In the case of severe neutropenia (<500 cells/mm 3 for seven days or more) during a course of paclitaxel protein-bound particles for injectable suspension (albumin-bound) therapy, reduce the dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in subsequent courses in patients with either MBC or NSCLC.
In patients with MBC, resume treatment with every-3-week cycles of paclitaxel protein-bound particles for injectable suspension (albumin-bound) after ANC recovers to a level >1,500 cells/mm 3 and platelets recover to a level >100,000 cells/mm 3. In patients with NSCLC, resume treatment if recommended at permanently reduced doses for both weekly paclitaxel protein-bound particles for injectable suspension (albumin-bound) and every-3-week carboplatin after ANC recovers to at least 1500 cells/mm 3 and platelet count of at least 100,000 cells/mm 3 on Day 1 or to an ANC of at least 500 cells/mm 3 and platelet count of at least 50,000 cells/mm 3 on Days 8 or 15 of the cycle [see Dosage and Administration (2.6)].
In patients with adenocarcinoma of the pancreas, withhold paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine if the ANC is less than 500 cells/mm 3 or platelets are less than 50,000 cells/mm 3 and delay initiation of the next cycle if the ANC is less than 1500 cells/mm 3 or platelet count is less than 100,000 cells/mm 3 on Day 1 of the cycle. Resume treatment with appropriate dose reduction if recommended [see Dosage and Administration (2.6)].
5.2 Severe Neuropathy
Sensory neuropathy is dose- and schedule-dependent [see Adverse Reactions (6.1)] . If ≥ Grade 3 sensory neuropathy develops, withhold paclitaxel protein-bound particles for injectable suspension (albumin-bound) treatment until resolution to Grade 1 or 2 for metastatic breast cancer or until resolution to ≤ Grade 1 for NSCLC and pancreatic cancer followed by a dose reduction for all subsequent courses of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Dosage and Administration (2.6)] .
5.3 Sepsis
Sepsis occurred in 5% of patients with or without neutropenia who received paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine. Biliary obstruction or presence of biliary stent were risk factors for severe or fatal sepsis.
If a patient becomes febrile (regardless of ANC) initiate treatment with broad spectrum antibiotics. For febrile neutropenia, interrupt paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine until fever resolves and ANC ≥ 1,500, then resume treatment at reduced dose levels [see Dosage and Administration (2.6)] .
5.4 Pneumonitis
Pneumonitis, including some cases that were fatal, occurred in 4% of patients receiving paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine.
Monitor patients for signs and symptoms of pneumonitis and interrupt paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine during evaluation of suspected pneumonitis. After ruling out infectious etiology and upon making a diagnosis of pneumonitis, permanently discontinue treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine.
5.5 Severe Hypersensitivity
Severe and sometimes fatal hypersensitivity reactions, including anaphylactic reactions, have been reported. Do not rechallenge patients who experience a severe hypersensitivity reaction to paclitaxel protein-bound particles for injectable suspension (albumin-bound) with this drug [see Contraindications (4)] .
Cross-hypersensitivity between paclitaxel protein-bound particles for injectable suspension (albumin-bound) and other taxane products has been reported and may include severe reactions such as anaphylaxis. Closely monitor patients with a previous history of hypersensitivity to other taxanes during initiation of paclitaxel protein-bound particles for injectable suspension (albumin-bound) therapy.
5.6 Use in Patients with Hepatic Impairment
The exposure and toxicity of paclitaxel can be increased in patients with hepatic impairment. Closely monitor patients with hepatic impairment for severe myelosuppression.
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is not recommended in patients who have total bilirubin >5 x ULN or AST >10 x ULN. In addition, paclitaxel protein-bound particles for injectable suspension (albumin-bound) is not recommended in patients with metastatic adenocarcinoma of the pancreas who have moderate to severe hepatic impairment (total bilirubin >1.5 x ULN and AST ≤10 x ULN). Reduce the starting dose for patients with moderate or severe hepatic impairment [see Dosage and Administration (2.5), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)] .
5.7 Albumin (Human)
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) contains albumin (human), a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries a remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob Disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
5.8 Embryo-Fetal Toxicity
Based on mechanism of action and findings in animals, paclitaxel protein-bound particles for injectable suspension (albumin-bound) can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of paclitaxel formulated as albumin-bound particles to rats during pregnancy at doses lower than the maximum recommended human dose, based on body surface area, caused embryo-fetal toxicities, including intrauterine mortality, increased resorptions, reduced numbers of live fetuses, and malformations.
Advise females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception and avoid becoming pregnant during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least six months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
Based on findings from genetic toxicity and animal reproduction studies, advise male patients with female partners of reproductive potential to use effective contraception and avoid fathering a child during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least three months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions (≥ 20%) with single-agent use of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in metastatic breast cancer are alopecia, neutropenia, sensory neuropathy, abnormal ECG, fatigue/asthenia, myalgia/arthralgia, AST elevation, alkaline phosphatase elevation, anemia, nausea, infections, and diarrhea [see Adverse Reactions (6.1)] .
The most common adverse reactions (≥ 20%) of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with carboplatin for non-small cell lung cancer are anemia, neutropenia, thrombocytopenia, alopecia, peripheral neuropathy, nausea, and fatigue [see Adverse Reactions (6.1)]. The most common serious adverse reactions of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with carboplatin for non-small cell lung cancer are anemia (4%) and pneumonia (3%). The most common adverse reactions resulting in permanent discontinuation of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are neutropenia (3%), thrombocytopenia (3%), and peripheral neuropathy (1%). The most common adverse reactions resulting in dose reduction of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are neutropenia (24%), thrombocytopenia (13%), and anemia (6%). The most common adverse reactions leading to withholding or delay in paclitaxel protein-bound particles for injectable suspension (albumin-bound) dosing are neutropenia (41%), thrombocytopenia (30%), and anemia (16%).
In a randomized open-label trial of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine for pancreatic adenocarcinoma [see Clinical Studies (14.3)], the most common (≥ 20%) selected (with a ≥ 5% higher incidence) adverse reactions of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are neutropenia, fatigue, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, pyrexia, vomiting, decreased appetite, rash, and dehydration [see Adverse Reactions (6.1)]. The most common serious adverse reactions of paclitaxel protein-bound particles for injectable suspension (albumin-bound) (with a ≥ 1% higher incidence) are pyrexia (6%), dehydration (5%), pneumonia (4%), and vomiting (4%). The most common adverse reactions resulting in permanent discontinuation of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are peripheral neuropathy (8%), fatigue (4%), and thrombocytopenia (2%). The most common adverse reactions resulting in dose reduction of paclitaxel protein-bound particles for injectable suspension (albumin-bound) are neutropenia (10%) and peripheral neuropathy (6%). The most common adverse reactions leading to withholding or delay in paclitaxel protein-bound particles for injectable suspension (albumin-bound) dosing are neutropenia (16%), thrombocytopenia (12%), fatigue (8%), peripheral neuropathy (15%), anemia (5%), and diarrhea (5%).
6.1 Clinical Trials Experience
Metastatic Breast Cancer
Table 6 shows the frequency of important adverse reactions in the randomized comparative trial for the patients who received either single-agent paclitaxel protein-bound particles for injectable suspension (albumin-bound) or paclitaxel injection for the treatment of metastatic breast cancer.
Table 6: Adverse Reactions in the Randomized Metastatic Breast Cancer Study on an Every-3-Weeks Schedule aPaclitaxel injection patients received premedication. bIncludes treatment-related events related to hypersensitivity (e.g., flushing, dyspnea, chest pain, hypotension) that began on a day of dosing. cSevere events are defined as at least Grade 3 toxicity. Percent of Patients Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound)
260 mg/m 2over 30 min
(n=229)Paclitaxel Injection
175 mg/m 2over 3 h a
(n=225)Bone Marrow Neutropenia < 2.0 x 10 9/L 80 82 < 0.5 x 10 9/L 9 22 Thrombocytopenia < 100 x 10 9/L 2 3 < 50 x 10 9/L <1 <1 Anemia < 11 g/dL 33 25 < 8 g/dL 1 <1 Infections 24 20 Febrile Neutropenia 2 1 Neutropenic Sepsis <1 <1 Bleeding 2 2 Hypersensitivity Reaction b All 4 12 Severe c 0 2 Cardiovascular Vital Sign Changes During Administration Bradycardia <1 <1 Hypotension 5 5 Severe Cardiovascular Events c 3 4 Abnormal ECG All Patients 60 52 Patients with Normal Baseline 35 30 Respiratory Cough 7 6 Dyspnea 12 9 Sensory Neuropathy Any Symptoms 71 56 Severe Symptoms c 10 2 Myalgia / Arthralgia Any Symptoms 44 49 Severe Symptoms c 8 4 Asthenia Any Symptoms 47 39 Severe Symptoms c 8 3 Fluid Retention/Edema Any Symptoms 10 8 Severe Symptoms c 0 <1 Gastrointestinal Nausea Any Symptoms 30 22 Severe Symptoms c 3 <1 Vomiting Any Symptoms 18 10 Severe Symptoms c 4 1 Diarrhea Any Symptoms 27 15 Severe Symptoms c <1 1 Mucositis Any Symptoms 7 6 Severe Symptoms c <1 0 Alopecia 90 94 Hepatic(Patients with Normal Baseline) Bilirubin Elevations 7 7 Alkaline Phosphatase Elevations 36 31 AST (SGOT) Elevations 39 32 Injection Site Reaction <1 1 Other Adverse Reactions
Hematologic Disorders
Neutropenia was dose dependent and reversible. Among patients with metastatic breast cancer in the randomized trial, neutrophil counts declined below 500 cells/mm 3(Grade 4) in 9% of the patients treated with a dose of 260 mg/m 2compared to 22% in patients receiving paclitaxel injection at a dose of 175 mg/m 2. Pancytopenia has been observed in clinical trials.Infections
Infectious episodes were reported in 24% of the patients treated with paclitaxel protein-bound particles for injectable suspension (albumin-bound). Oral candidiasis, respiratory tract infections and pneumonia were the most frequently reported infectious complications.Hypersensitivity Reactions (HSRs)
Grade 1 or 2 HSRs occurred on the day of paclitaxel protein-bound particles for injectable suspension (albumin-bound) administration and consisted of dyspnea (1%) and flushing, hypotension, chest pain, and arrhythmia (all <1%). The use of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in patients previously exhibiting hypersensitivity to paclitaxel injection or human albumin has not been studied.Cardiovascular
Hypotension, during the 30-minute infusion, occurred in 5% of patients. Bradycardia, during the 30-minute infusion, occurred in <1% of patients. These vital sign changes most often caused no symptoms and required neither specific therapy nor treatment discontinuation.Severe cardiovascular events possibly related to single-agent paclitaxel protein-bound particles for injectable suspension (albumin-bound) occurred in approximately 3% of patients. These events included cardiac ischemia/infarction, chest pain, cardiac arrest, supraventricular tachycardia, edema, thrombosis, pulmonary thromboembolism, pulmonary emboli, and hypertension. Cases of cerebrovascular attacks (strokes) and transient ischemic attacks have been reported.
Electrocardiogram (ECG) abnormalities were common among patients at baseline. ECG abnormalities on study did not usually result in symptoms, were not dose-limiting, and required no intervention. ECG abnormalities were noted in 60% of patients. Among patients with a normal ECG prior to study entry, 35% of all patients developed an abnormal tracing while on study. The most frequently reported ECG modifications were non-specific repolarization abnormalities, sinus bradycardia, and sinus tachycardia.
Respiratory
Dyspnea (12%), cough (7%), and pneumothorax (<1%) were reported after treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound).Neurologic
The frequency and severity of sensory neuropathy increased with cumulative dose. Sensory neuropathy was the cause of paclitaxel protein-bound particles for injectable suspension (albumin-bound) discontinuation in 7/229 (3%) patients. Twenty-four patients (10%) treated with paclitaxel protein-bound particles for injectable suspension (albumin-bound) developed Grade 3 peripheral neuropathy; of these patients, 14 had documented improvement after a median of 22 days; 10 patients resumed treatment at a reduced dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) and 2 discontinued due to peripheral neuropathy. Of the 10 patients without documented improvement, 4 discontinued the study due to peripheral neuropathy.No Grade 4 sensory neuropathies were reported. Only one incident of motor neuropathy (Grade 2) was observed in either arm of the controlled trial.
Vision Disorders
Ocular/visual disturbances occurred in 13% of all patients (n=366) treated with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and 1% were severe. The severe cases (keratitis and blurred vision) were reported in patients who received higher doses than those recommended (300 or 375 mg/m 2). These effects generally have been reversible.Arthralgia/Myalgia
The symptoms were usually transient, occurred two or three days after paclitaxel protein-bound particles for injectable suspension (albumin-bound) administration, and resolved within a few days.Hepatic
Grade 3 or 4 elevations in GGT were reported for 14% of patients treated with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and 10% of patients treated with paclitaxel injection in the randomized trial.Renal
Overall 11% of patients experienced creatinine elevation, 1% severe. No discontinuations, dose reductions, or dose delays were caused by renal toxicities.Other Clinical Events
Nail changes (changes in pigmentation or discoloration of nail bed) have been reported. Edema occurred in 10% of patients; no patients had severe edema. Dehydration and pyrexia were also reported.Non-Small Cell Lung Cancer
Adverse reactions were assessed in 514 paclitaxel protein-bound particles for injectable suspension (albumin-bound)/carboplatin-treated patients and 524 paclitaxel injection/carboplatin-treated patients receiving first-line systemic treatment for locally advanced (stage IIIB) or metastatic (IV) non-small cell lung cancer (NSCLC) in a multicenter, randomized, open-label trial. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) was administered as an intravenous infusion over 30 minutes at a dose of 100 mg/m 2 on Days 1, 8, and 15 of each 21-day cycle. Paclitaxel injection was administered as an intravenous infusion over 3 hours at a dose of 200 mg/m 2, following premedication. In both treatment arms carboplatin at a dose of AUC = 6 mgmin/mL was administered intravenously on Day 1 of each 21-day cycle after completion of paclitaxel protein-bound particles for injectable suspension (albumin-bound)/paclitaxel infusion.
The differences in paclitaxel dose and schedule between the two arms limit direct comparison of dose-and schedule-dependent adverse reactions. Among patients evaluable for adverse reactions, the median age was 60 years, 75% were men, 81% were White, 49% had adenocarcinoma, 43% had squamous cell lung cancer, 76% were ECOG PS 1. Patients in both treatment arms received a median of 6 cycles of treatment.
The following common (≥ 10% incidence) adverse reactions were observed at a similar incidence in paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus carboplatintreated and paclitaxel injection plus carboplatin-treated patients: alopecia 56%, nausea 27%, fatigue 25%, decreased appetite 17%, asthenia 16%, constipation 16%, diarrhea 15%, vomiting 12%, dyspnea 12%, and rash 10% (incidence rates are for the paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus carboplatin treatment group).
Table 7 provides the frequency and severity of laboratory-detected abnormalities which occurred with a difference of ≥5% for all grades (1-4) or≥2% for Grade 3-4 toxicity between paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus carboplatin-treated patients or paclitaxel injection plus carboplatin-treated patients.
Table 7: Selected Hematologic Laboratory-Detected Abnormalities with a Difference of ≥ 5% for grades (1-4) or ≥ 2% for Grade 3-4 Toxicity Between Treatment Groups Paclitaxel protein-bound particles for injectable suspension (albumin-bound) (100 mg/m 2 weekly) plus carboplatin Paclitaxel Injection (200 mg/m 2 every 3 weeks) plus carboplatin Grades 1-4 (%) Grade 3-4 (%) Grades 1-4 (%) Grade 3-4 (%) Anemia 1,2
98 28 91 7 Neutropenia 1,3 85 47 83 58 Thrombocytopenia 1,3 68 18 55 9 1 508 patients assessed in Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/carboplatin-treated group.
2 514 patients assessed in paclitaxel injection/carboplatin-treated group.
3 513 patients assessed in paclitaxel injection/carboplatin-treated group.
Table 8 provides the frequency and severity of adverse reactions, which occurred with a difference of ≥ 5% for all grades (1-4) or ≥ 2% for Grade 3-4 between either treatment group for the 514 Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus carboplatin-treated patients compared with the 524 patients who received paclitaxel injection plus carboplatin.
Table 8: Selected Adverse Reactions with a Difference of ≥5% for All Grade Toxicity or ≥2% for Grade 3-4 Toxicity Between Treatment Groups System Organ Class Adverse Reaction Paclitaxel protein-bound particles for injectable suspension (albumin-bound) (100 mg/m 2 weekly) + carboplatin (N=514) Paclitaxel Injection (200 mg/m 2 every 3 weeks) + carboplatin (N=524) Grade 1-4 Toxicity (%) Grade 3-4 Toxicity (%) Grades 1-4 Toxicity (%) Grade 3-4 Toxicity (%) Nervous system disorders Peripheral neuropathy a 48 3 64 12 General disorders and administration site conditions Edema peripheral 10 0 4 <1 Respiratory thoracic and mediastinal disorders Epistaxis 7 0 2 0 Musculoskeletal and connective tissue disorders Arthralgia 13 <1 25 2 Myalgia 10 <1 19 2 a Peripheral neuropathy is defined by the MedDRA Version 14.0 SMQ neuropathy (broad scope). For the Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus carboplatin treated group, 17/514 (3%) patients developed Grade 3 peripheral neuropathy and no patients developed Grade 4 peripheral neuropathy. Grade 3 neuropathy improved to Grade 1 or resolved in 10/17 patients (59%) following interruption or discontinuation of Paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Adenocarcinoma of the Pancreas
Adverse reactions were assessed in 421 patients who received Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus gemcitabine and 402 patients who received gemcitabine for the first-line systemic treatment of metastatic adenocarcinoma of the pancreas in a multicenter, multinational, randomized, controlled, open-label trial. Patients received a median treatment duration of 3.9 months in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine group and 2.8 months in the gemcitabine group. For the treated population, the median relative dose intensity for gemcitabine was 75% in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine group and 85% in the gemcitabine group. The median relative dose intensity of Paclitaxel protein-bound particles for injectable suspension (albumin-bound) was 81%.
Table 9 provides the frequency and severity of laboratory-detected abnormalities which occurred at a higher incidence for Grades 14 (≥ 5%) or for Grade 3-4 (≥ 2%) toxicity in Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus gemcitabine-treated patients.
Table 9: Selected Hematologic Laboratory-Detected Abnormalities with a Higher Incidence (≥ 5% for Grades 1-4 or≥ 2% for Grades 3-4 Events) in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/Gemcitabine Arm Paclitaxel protein-bound particles for injectable suspension (albumin-bound) (125 mg/m 2)/Gemcitabine d Gemcitabine Grades 1-4 (%) Grades 3-4 (%) Grades 1-4 (%) Grades 3-4 (%) Neutropenia a,b 73 38 58 27 Thrombocytopenia b,c 74 13 70 9 a 405 patients assessed in Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine-treated group.
b 388 patients assessed in gemcitabine-treated group. c 404 patients assessed in Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine-treated group. d Neutrophil growth factors were administered to 26% of patients in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine group. Table10 provides the frequency and severity of adverse reactions which occurred with a difference of ≥ 5% for all grades or ≥ 2% for Grade 3 or higher in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus gemcitabine-treated group compared to the gemcitabine group.
Table 10: Selected Adverse Reactions with a Higher Incidence (≥5% for All Grade Toxicity or ≥2% for Grade 3 or Higher Toxicity) in the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/Gemcitabine Arm System Organ Class Adverse Reaction Paclitaxel protein-bound particles for injectable suspension (albumin-bound) (125 mg/m 2) and gemcitabine (N=421) Gemcitabine (N=402) All Grades Grade 3 or Higher All Grades Grade 3 or Higher General disorders and administration site conditions Fatigue 248 (59%) 77 (18%) 183 (46%) 37 (9%) Peripheral edema 194 (46%) 13 (3%) 122 (30%) 12 (3%) Pyrexia 171 (41%) 12 (3%) 114 (28%) 4 (1%) Asthenia 79 (19%) 29 (7%) 54 (13%) 17 (4%) Mucositis 42 (10%) 6 (1%) 16 (4%) 1 (<1%) Gastrointestinal disorders Nausea 228 (54%) 27 (6%) 192 (48%) 14 (3%) Diarrhea 184 (44%) 26 (6%) 95 (24%) 6 (1%) Vomiting 151 (36%) 25 (6%) 113 (28%) 15 (4%) Alopecia 212 (50%) 6 (1%) 21 (5%) 0 Skin and subcutaneous tissue disorders Rash 128 (30%) 8 (2%) 45 (11%) 2 (<1%) Nervous system disorders Peripheral neuropathya 227 (54%) 70 (17%) 51 (13%) 3 (1%) Dysgeusia 68 (16%) 0 33 (8%) 0 Headache 60 (14%) 1 (<1%) 38 (9%) 1 (<1%) Metabolism and nutrition disorders Decreased appetite 152 (36%) 23 (5%) 104 (26%) 8 (2%) Dehydration 87 (21%) 31 (7%) 45 (11%) 10 (2%) Hypokalemia 52 (12%) 18 (4%) 28 (7%) 6 (1%) Respiratory, thoracic and mediastinal disorders Cough 72 (17%) 0 30 (7%) 0 Epistaxis 64 (15%) 1 (<1%) 14 (3%) 1 (<1%) Infections and infestations Urinary tract infections b 47 (11%) 10 (2%) 20 (5%) 1 (<1%) Musculoskeletal and connective tissue disorders Pain in extremity 48 (11%) 3 (1%) 24 (6%) 3 (1%) Arthralgia 47 (11%) 3 (1%) 13 (3%) 1 (<1%) Myalgia 44 (10%) 4 (1%) 15 (4%) 0 Psychiatric disorders Depression 51 (12%) 1 (<1%) 24 (6%) 0 a Peripheral neuropathy is defined by the MedDRA Version 15.0 Standard MedDRA Query neuropathy (broad scope).
b Urinary tract infections includes the preferred terms of: urinary tract infection, cystitis, urosepsis, urinary tract infection bacterial, and urinary tract infection enterococcal.
Additional clinically relevant adverse reactions that were reported in < 10% of the patients with adenocarcinoma of the pancreas who received paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine included:
Infections & infestations: oral candidiasis, pneumonia
Vascular disorders: hypertension
Cardiac disorders: tachycardia, congestive cardiac failure
Eye disorders: cystoid macular edema
Peripheral Neuropathy
Grade 3 peripheral neuropathy occurred in 17% of patients who received paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine compared to 1% of patients who received gemcitabine only; no patients developed Grade 4 peripheral neuropathy. The median time to first occurrence of Grade 3 peripheral neuropathy in the paclitaxel protein-bound particles for injectable suspension (albumin-bound) arm was 140 days. Upon suspension of paclitaxel protein-bound particles for injectable suspension (albumin-bound) dosing, the median time to improvement from Grade 3 peripheral neuropathy to ≤ Grade 1 was 29 days. Of paclitaxel protein-bound particles for injectable suspension (albumin-bound)-treated patients with Grade 3 peripheral neuropathy, 44% resumed paclitaxel protein-bound particles for injectable suspension (albumin-bound) at a reduced dose.
Sepsis
Sepsis occurred in 5% of patients who received paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine compared to 2% of patients who received gemcitabine alone. Sepsis occurred both in patients with and without neutropenia. Risk factors for sepsis included biliary obstruction or presence of biliary stent.
Pneumonitis
Pneumonitis occurred in 4% of patients who received paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine compared to 1% of patients who received gemcitabine alone. Two of 17 patients in the paclitaxel protein-bound particles for injectable suspension (albumin-bound) arm with pneumonitis died.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of paclitaxel protein-bound particles for injectable suspension (albumin-bound) or with paclitaxel injection and may be expected to occur with paclitaxel protein-bound particles for injectable suspension (albumin-bound). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity Reactions
Severe and sometimes fatal hypersensitivity reactions. Cross-hypersensitivity between paclitaxel protein-bound particles for injectable suspension (albumin-bound) and other taxanes has been reported.
Cardiovascular
Congestive heart failure, left ventricular dysfunction, and atrioventricular block. Most patients were previously exposed to cardiotoxic drugs, such as anthracyclines, or had underlying cardiac history.
Respiratory
Pneumonitis, interstitial pneumonia, and pulmonary embolism
Radiation pneumonitis in patients receiving concurrent radiotherapy.
Lung fibrosis has been reported with paclitaxel injection.
Neurologic
Cranial nerve palsies and vocal cord paresis, as well as autonomic neuropathy resulting in paralytic ileus.
Vision Disorders
Reduced visual acuity due to cystoid macular edema (CME). After cessation of treatment, CME may improve, and visual acuity may return to baseline. Abnormal visual evoked potentials in patients treated with paclitaxel injection suggest persistent optic nerve damage.
Hepatic
Hepatic necrosis and hepatic encephalopathy leading to death in patients treated with paclitaxel injection.
Gastrointestinal (GI)
Intestinal obstruction, intestinal perforation, pancreatitis, and ischemic colitis. In patients treated with paclitaxel injection, neutropenic enterocolitis (typhlitis) despite the coadministration of G-CSF, alone and in combination with other chemotherapeutic agents.
Injection Site Reaction
Extravasation. Closely monitor the paclitaxel protein-bound particles for injectable suspension (albumin-bound) infusion site for possible infiltration during drug administration [see Dosage and Administration 2.1)] .
Severe events such as phlebitis, cellulitis, induration, necrosis, and fibrosis have been reported with paclitaxel injection. In some cases, the onset of the injection site reaction occurred during a prolonged infusion or was delayed up to ten days. Recurrence of skin reactions at a site of previous extravasation following administration of paclitaxel injection at a different site has been reported.
Metabolic and Nutritional Disorders
Tumor lysis syndrome
Other Clinical Events
Skin reactions including generalized or maculopapular rash, erythema, and pruritus
Photosensitivity reactions, radiation recall phenomenon, scleroderma, and in some patients previously exposed to capecitabine, reports of palmar-plantar erythrodysesthesia. Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.
Conjunctivitis, cellulitis, and increased lacrimation have been reported with paclitaxel injection.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings in animals, paclitaxel protein-bound particles for injectable suspension (albumin-bound) can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] . There are no available human data on paclitaxel protein-bound particles for injectable suspension (albumin-bound) use in pregnant women to inform the drug-associated risk.
In animal reproduction studies, administration of paclitaxel formulated as albumin-bound particles to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity at doses approximately 2% of the daily maximum recommended human dose on a mg/m 2basis (see Data). Advise females of reproductive potential of the potential risk to a fetus.
The background rate of major birth defects and miscarriage is unknown for the indicated population. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryo-fetal development studies, intravenous administration of paclitaxel formulated as albumin-bound particles to rats during pregnancy, on gestation days 7 to 17 at doses of 6 mg/m 2(approximately 2% of the daily maximum recommended human dose on a mg/m 2basis) caused embryo-fetal toxicities, as indicated by intrauterine mortality, increased resorptions (up to 5-fold), reduced numbers of litters and live fetuses, reduction in fetal body weight, and increase in fetal anomalies. Fetal anomalies included soft tissue and skeletal malformations, such as eye bulge, folded retina, microphthalmia, and dilation of brain ventricles.
8.2 Lactation
Risk Summary
There are no data on the presence of paclitaxel in human milk, or its effect on the breastfed child or on milk production. In animal studies, paclitaxel and/or its metabolites were excreted into the milk of lactating rats (see Data). Because of the potential for serious adverse reactions in a breastfed child from paclitaxel protein-bound particles for injectable suspension (albumin-bound), advise lactating women not to breastfeed during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for two weeks after the last dose.
8.3 Females and Males of Reproductive Potential
Based on animal studies and mechanism of action, paclitaxel protein-bound particles for injectable suspension (albumin-bound) can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to starting treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Contraception
Females
Advise females of reproductive potential to use effective contraception and avoid becoming pregnant during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least six months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Males
Based on findings in genetic toxicity and animal reproduction studies, advise males with female partners of reproductive potential to use effective contraception and avoid fathering a child during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least three months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Use in Specific Populations (8.1)and Nonclinical Toxicology (13.1)].
Infertility
Females and Males
Based on findings in animals, paclitaxel protein-bound particles for injectable suspension (albumin-bound) may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Pharmacokinetics, safety, and antitumor activity of paclitaxel protein-bound particles for injectable suspension (albumin-bound) were assessed in an open-label, dose escalation, dose expansion study ( NCT01962103) in 96 pediatric patients aged 1.4 to < 17 years with recurrent or refractory pediatric solid tumors. The maximum tolerated dose (MTD) normalized for body surface area (BSA) was lower in pediatric patients compared to adults. No new safety signals were observed in pediatric patients across these studies.
Paclitaxel protein-bound exposures normalized by dose were higher in 96 pediatric patients (aged 1.4 to < 17 years) as compared to those in adults.
8.5 Geriatric Use
Of the 229 patients in the randomized study who received paclitaxel protein-bound particles for injectable suspension (albumin-bound) for the treatment of metastatic breast cancer, 13% were at least 65 years of age and < 2% were 75 years or older. This study of paclitaxel protein-bound particles for injectable suspension (albumin-bound) did not include a sufficient number of patients with metastatic breast cancer who were 65 years and older to determine whether they respond differently from younger patients.
A subsequent pooled analysis was conducted in 981 patients receiving paclitaxel protein-bound particles for injectable suspension (albumin-bound) monotherapy for metastatic breast cancer, of which 15% were 65 years of age or older and 2% were 75 years of age or older. A higher incidence of epistaxis, diarrhea, dehydration, fatigue, and peripheral edema was found in patients 65 years of age or older.
Of the 514 patients in the randomized study who received paclitaxel protein-bound particles for injectable suspension (albumin-bound) and carboplatin for the first-line treatment of non-small cell lung cancer, 31% were 65 years or older and 3.5% were 75 years or older. Myelosuppression, peripheral neuropathy, and arthralgia were more frequent in patients 65 years or older compared to patients younger than 65 years old. No overall difference in effectiveness, as measured by response rates, was observed between patients 65 years or older compared to patients younger than 65 years old.
Of the 431 patients in the randomized study who received paclitaxel protein-bound particles for injectable suspension (albumin-bound) and gemcitabine for the first-line treatment of pancreatic adenocarcinoma, 41% were 65 years or older and 10% were 75 years or older. No overall differences in effectiveness were observed between patients who were 65 years of age or older and younger patients. Diarrhea, decreased appetite, dehydration, and epistaxis were more frequent in patients 65 years or older compared with patients younger than 65 years old. Clinical studies of paclitaxel protein-bound particles for injectable suspension (albumin-bound) did not include sufficient number of patients with pancreatic cancer who were 75 years and older to determine whether they respond differently from younger patients.
8.6 Renal Impairment
No adjustment of the starting paclitaxel protein-bound particles for injectable suspension (albumin-bound) dose is required for patients with mild to moderate renal impairment (estimated creatinine clearance 30 to <90 mL/min) [see Clinical Pharmacology (12.3)]. There are insufficient data to permit dosage recommendations in patients with severe renal impairment or end stage renal disease (estimated creatinine clearance <30 mL/min).
8.7 Hepatic Impairment
No adjustment of the starting paclitaxel protein-bound particles for injectable suspension (albumin-bound) dose is required for patients with mild hepatic impairment (total bilirubin > ULN and ≤ 1.5 x ULN and aspartate aminotransferase [AST] ≤ 10 x ULN). Reduce paclitaxel protein-bound particles for injectable suspension (albumin-bound) starting dose in patients with moderate to severe hepatic impairment [see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)] . Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is not recommended for use in patients with total bilirubin > 5 x ULN or AST > 10 x ULN [see Dosage and Administration (2.5), Warnings and Precautions (5.6), and Clinical Pharmacology (12.3)] . Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is not recommended for use in patients with metastatic adenocarcinoma of the pancreas who have moderate to severe hepatic impairment [see Dosage and Administration (2.5)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is paclitaxel formulated as albumin-bound nanoparticles with a mean particle size of approximately 130 nanometers. Paclitaxel exists in the particles in a non-crystalline, amorphous state. Paclitaxel is a microtubule inhibitor. The chemical name for paclitaxel is 5β,20-Epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2 R,3 S)- N-benzoyl-3-phenylisoserine. The empirical formula is C 47H 51NO 14and the molecular weight is 853.92. Paclitaxel has the following structural formula:
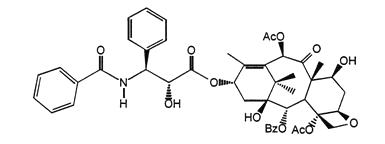
Paclitaxel is a white to off-white crystalline powder. It is highly lipophilic, insoluble in water, and melts at approximately 213°C to 216°C.
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is supplied as a white to yellow, sterile, lyophilized powder for reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP prior to intravenous infusion. Each single‑dose vial contains 100 mg of paclitaxel (bound to human albumin) and approximately 900 mg of human albumin (containing sodium caprylate and sodium acetyltryptophanate). Each milliliter (mL) of reconstituted suspension contains 5 mg paclitaxel formulated as albumin‑bound particles. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is free of solvents.
-
12 CLINICAL PHARMACOLOGY
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is a microtubule inhibitor that promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization. This stability results in the inhibition of the normal dynamic reorganization of the microtubule network that is essential for vital interphase and mitotic cellular functions. Paclitaxel induces abnormal arrays or "bundles" of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis.
12.3 Pharmacokinetics
The pharmacokinetics of total paclitaxel following 30- and 180-minute infusions of paclitaxel protein-bound particles for injectable suspension (albumin-bound) at dose levels of 80 to 375 mg/m 2(0.31 to 1.15 times the maximum approved recommended dosage) were determined in clinical studies. Dose levels of mg/m 2refer to mg of paclitaxel in paclitaxel protein-bound particles for injectable suspension (albumin-bound). Following intravenous administration of paclitaxel protein-bound particles for injectable suspension (albumin-bound) to patients with solid tumors, paclitaxel plasma concentrations declined in a biphasic manner, the initial rapid decline representing distribution to the peripheral compartment and the slower second phase representing drug elimination.
Following paclitaxel protein-bound particles for injectable suspension (albumin-bound) infusion, paclitaxel exhibited linear drug exposure (AUC) across clinical doses ranging from 80 to 300 mg/m 2(0.31 to 1.15 times the maximum approved recommended dosage). The pharmacokinetics of paclitaxel in paclitaxel protein-bound particles for injectable suspension (albumin-bound) were independent of the duration of intravenous administration.
The pharmacokinetic data of 260 mg/m 2 paclitaxel protein-bound particles for injectable suspension (albumin-bound) administered over a 30-minute infusion was compared to the pharmacokinetics of 175 mg/m 2paclitaxel injection over a 3-hour infusion. Clearance was larger (43%) and the volume of distribution was higher (53%) for paclitaxel protein-bound particles for injectable suspension (albumin-bound) than for paclitaxel injection. There were no differences in terminal half-lives.
Distribution
Following paclitaxel protein-bound particles for injectable suspension (albumin-bound) administration to patients with solid tumors, paclitaxel is evenly distributed into blood cells and plasma and is highly bound to plasma proteins (94%). The total volume of distribution is approximately 1741 L; the large volume of distribution indicates extensive extravascular distribution and/or tissue binding of paclitaxel.
In a within-patient comparison study, the fraction of unbound paclitaxel in plasma was significantly higher with paclitaxel protein-bound particles for injectable suspension (albumin-bound) (6.2%) than with solvent-based paclitaxel (2.3%). This contributes to significantly higher exposure to unbound paclitaxel with paclitaxel protein-bound particles for injectable suspension (albumin-bound) compared with solvent-based paclitaxel, when the total exposure is comparable. In vitrostudies of binding to human serum proteins, using paclitaxel concentrations ranging from 0.1 to 50 µg/mL, indicated that the presence of cimetidine, ranitidine, dexamethasone, or diphenhydramine did not affect protein binding of paclitaxel.
Elimination
At the clinical dose range of 80 to 300 mg/m 2(0.31 to 1.15 times the maximum approved recommended dosage), the mean total clearance of paclitaxel ranges from 13 to 30 L/h/m 2and the mean terminal half-life ranges from 13 to 27 hours.Metabolism
In vitrostudies with human liver microsomes and tissue slices showed that paclitaxel in paclitaxel protein-bound particles for injectable suspension (albumin-bound) was metabolized primarily to 6α-hydroxypaclitaxel by CYP2C8; and to two minor metabolites, 3'- p-hydroxypaclitaxel and 6α, 3'- p-dihydroxypaclitaxel, by CYP3A4. In vitro, the metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents (ketoconazole, verapamil, diazepam, quinidine, dexamethasone, cyclosporin, teniposide, etoposide, and vincristine), but the concentrations used exceeded those found in vivofollowing normal therapeutic doses. Testosterone, 17α-ethinyl estradiol, retinoic acid, and quercetin, a specific inhibitor of CYP2C8, also inhibited the formation of 6α-hydroxypaclitaxel in vitro. The pharmacokinetics of paclitaxel may also be altered in vivoas a result of interactions with compounds that are substrates, inducers, or inhibitors of CYP2C8 and/or CYP3A4 [see Drug Interactions (7)] .Excretion
After a 30-minute infusion of 260 mg/m 2doses of paclitaxel protein-bound particles for injectable suspension (albumin-bound), the mean values for cumulative urinary recovery of unchanged drug (4%) indicated extensive non-renal clearance. Less than 1% of the total administered dose was excreted in urine as the metabolites 6α-hydroxypaclitaxel and 3'- p-hydroxypaclitaxel. Fecal excretion was approximately 20% of the total dose administered.Specific Populations
No clinically meaningful differences in the pharmacokinetics of paclitaxel in paclitaxel protein-bound particles for injectable suspension (albumin-bound) were observed based on body weight (40 to 143 kg), body surface area (1.3 to 2.4 m 2), sex, race (Asian vs. White), age (24 to 85 years), type of solid tumors, mild to moderate renal impairment (creatinine clearance 30 to <90 mL/min), and mild hepatic impairment (total bilirubin >1 to ≤1.5 x ULN and AST ≤10 x ULN).
Patients with moderate (total bilirubin >1.5 to 3 x ULN and AST ≤10 x ULN) or severe (total bilirubin >3 to 5 x ULN) hepatic impairment had a 22% to 26% decrease in the maximum elimination rate of paclitaxel and approximately 20% increase in mean paclitaxel AUC compared with patients with normal hepatic function (total bilirubin ≤ULN and AST ≤ULN) [see Dosage and Administration (2.5)and Use in Specific Populations (8.7)] .
The effect of severe renal impairment or end stage renal disease (creatinine clearance < 30 mL/min) on the pharmacokinetics of paclitaxel in paclitaxel protein-bound particles for injectable suspension (albumin-bound) is unknown.
Drug Interaction Studies
Carboplatin: Administration of carboplatin immediately after the completion of the paclitaxel protein-bound particles for injectable suspension (albumin-bound) infusion to patients with NSCLC did not cause clinically meaningful changes in paclitaxel exposure. The observed mean AUC inf of free carboplatin was approximately 23% higher than the targeted value (6 min*mg/mL), but its mean half-life and clearance were consistent with those reported in the absence of paclitaxel.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of paclitaxel protein-bound particles for injectable suspension (albumin-bound) has not been studied.
Paclitaxel was clastogenic in vitro(chromosome aberrations in human lymphocytes) and in vivo(micronucleus test in mice). Paclitaxel was not mutagenic in the Ames test or the CHO/HGPRT gene mutation assay.
Administration of paclitaxel formulated as albumin-bound particles to male rats at 42 mg/m 2on a weekly basis (approximately 16% of the daily maximum recommended human exposure on a body surface area basis) for 11 weeks prior to mating with untreated female rats resulted in significantly reduced fertility accompanied by decreased pregnancy rates and increased loss of embryos in mated females. A dose of 42 mg/m 2also reduced male reproductive organ weights, mating performance, and sperm production. Testicular atrophy/degeneration was observed in single-dose toxicology studies in animals administered paclitaxel formulated as albumin-bound particles at doses lower than the recommended human dose; doses were 54 mg/m 2in rodents and 175 mg/m 2in dogs. Similar testicular degeneration was seen in monkeys administered three weekly doses of 108 mg/m 2paclitaxel formulated as albumin bound particles.
Administration of paclitaxel prior to and during mating produced impairment of fertility in male and female rats. Paclitaxel caused reduced fertility and reproductive indices, and increased embryo-fetal toxicity.
-
14 CLINICAL STUDIES
14 CLINICAL STUDIES
14.1 Metastatic Breast Cancer
Data from 106 patients accrued in two single arm open label studies and from 460 patients enrolled in a randomized comparative study were available to support the use of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in metastatic breast cancer.
Single Arm Open Label Studies
In one study, paclitaxel protein-bound particles for injectable suspension (albumin-bound) was administered as a 30-minute infusion at a dose of 175 mg/m 2to 43 patients with metastatic breast cancer. The second trial utilized a dose of 300 mg/m 2as a 30-minute infusion in 63 patients with metastatic breast cancer. Cycles were administered at 3-week intervals. Objective responses were observed in both studies.Randomized Comparative Study
This multicenter trial was conducted in 460 patients with metastatic breast cancer. Patients were randomized to receive paclitaxel protein-bound particles for injectable suspension (albumin-bound) at a dose of 260 mg/m 2given as a 30-minute infusion, or paclitaxel injection at 175 mg/m 2given as a 3-hour infusion. Sixty-four percent of patients had impaired performance status (ECOG 1 or 2) at study entry; 79% had visceral metastases; and 76% had > 3 sites of metastases. Fourteen percent of the patients had not received prior chemotherapy; 27% had received chemotherapy in the adjuvant setting, 40% in the metastatic setting and 19% in both metastatic and adjuvant settings. Fifty-nine percent received study drug as second or greater than second-line therapy. Seventy-seven percent of the patients had been previously exposed to anthracyclines.In this trial, patients in the paclitaxel protein-bound particles for injectable suspension (albumin-bound) treatment arm had a statistically significantly higher reconciled target lesion response rate (the trial primary endpoint) of 21.5% (95% CI: 16.2% to 26.7%), compared to 11.1% (95% CI: 6.9% to 15.1%) for patients in the paclitaxel injection treatment arm. See Table 11. There was no statistically significant difference in overall survival between the two study arms.
Table 11: Efficacy Results from Randomized Metastatic Breast Cancer Trial aReconciled Target Lesion Response Rate (TLRR) was the prospectively defined protocol specific endpoint, based on independent radiologic assessment of tumor responses reconciled with investigator responses (which also included clinical information) for the first 6 cycles of therapy. The reconciled TLRR was lower than the investigator Reported Response Rates, which are based on all cycles of therapy. bFrom Cochran-Mantel-Haenszel test stratified by 1 stline vs. > 1 stline therapy. cPrior therapy included an anthracycline unless clinically contraindicated. Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound)
260 mg/m 2Paclitaxel Injectionn
175 mg/m 2Reconciled Target Lesion Response Rate (primary endpoint) a All randomized patients Response Rate
[95% CI]50/233 (21.5%)
[16.19% to 26.73%]25/227 (11.1%)
[6.94% to 15.09%]p-value b 0.003 Patients who had failed combination chemotherapy or relapsed within 6 months of adjuvant chemotherapy c Response Rate
[95% CI]20/129 (15.5%)
[9.26% to 21.75%]12/143 (8.4%)
[3.85% to 12.94%]14.2 Non-Small Cell Lung Cancer
A multicenter, randomized, open-label study was conducted in 1052 chemotherapy naive patients with Stage IIIb/IV non-small cell lung cancer to compare Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound) in combination with carboplatin to paclitaxel injection in combination with carboplatin as first-line treatment in patients with advanced non-small cell lung cancer. Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound) was administered as an intravenous infusion over 30 minutes at a dose of 100 mg/m2 on Days 1, 8, and 15 of each 21-day cycle. Paclitaxel injection was administered as an intravenous infusion over 3 hours at a dose of 200 mg/m 2, following premedication. In both treatment arms carboplatin at a dose of AUC = 6 mgmin/mL was administered intravenously on Day 1 of each 21-day cycle after completion of Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound)/paclitaxel infusion. Treatment was administered until disease progression or development of an unacceptable toxicity. The major efficacy outcome measure was overall response rate as determined by a central independent review committee using RECIST guidelines (Version 1.0).
In the intent-to-treat (all-randomized) population, the median age was 60 years, 75% were men, 81% were White, 49% had adenocarcinoma, 43% had squamous cell lung cancer, 76% were ECOG PS 1, and 73% were current or former smokers. Patients received a median of 6 cycles of treatment in both study arms.
Patients in the Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound)/carboplatin arm had a statistically significantly higher overall response rate compared to patients in the paclitaxel injection/carboplatin arm [(33% versus 25%) see Table 12]. There was no statistically significant difference in overall survival between the two study arms.Table 12: Efficacy Results from Randomized Non-Small Cell Lung Cancer Trial (Intent-to-Treat Population) Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound) (100 mg/m 2 weekly) + carboplatin (N=521) Paclitaxel Injection
(200 mg/m 2 every 3 weeks) + carboplatin
(N=531)Overall Response Rate (ORR) Confirmed complete or partial overall response, n (%) 170 (33%) 132 (25%) 95% CI 28.6, 36.7 21.2, 28.5 P-value (Chi-Square test) 0.005 Median DoR in months (95% CI) 6.9 (5.6, 8.0) 6.0 (5.6, 7.1) Overall Response Rate by Histology Carcinoma/Adenocarcinoma 66/254 (26%) 71/264 (27%) Squamous Cell Carcinoma 94/229 (41%) 54/221 (24%) Large Cell Carcinoma 3/9 (33%) 2/13 (15%) Other 7/29 (24%) 5/33 (15%) 14.3 Adenocarcinoma of the Pancreas
A multicenter, multinational, randomized, open-label study was conducted in 861 patients comparing Paclitaxel protein-bound particles for injectable suspension (albumin-bound) plus gemcitabine versus gemcitabine monotherapy as first-line treatment of metastatic adenocarcinoma of the pancreas. Key eligibility criteria were Karnofsky Performance Status (KPS) ≥70, normal bilirubin level, transaminase levels ≤ 2.5 times the upper limit of normal (ULN) or ≤ 5 times the ULN for patients with liver metastasis, no prior cytotoxic chemotherapy in the adjuvant setting or for metastatic disease, no ongoing active infection requiring systemic therapy, and no history of interstitial lung disease. Patients with rapid decline in KPS (≥10%) or serum albumin (≥20%) during the 14 day screening period prior to study randomization were ineligible.
A total of 861 patients were randomized (1:1) to the Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine arm (N=431) or to the gemcitabine arm (N=430).
Randomization was stratified by geographic region (Australia, Western Europe, Eastern Europe, or North America), KPS (70 to 80 versus 90 to 100), and presence of liver metastasis (yes versus no). Patients randomized to Paclitaxel protein-bound particles for injectable suspension (albumin-bound)/gemcitabine received Paclitaxel protein-bound particles for injectable suspension (albumin-bound) 125 mg/m 2 as an intravenous infusion over 30-40 minutes followed by gemcitabine 1000 mg/m2 as an intravenous infusion over 30-40 minutes on Days 1, 8, and 15 of each 28-day cycle. Patients randomized to gemcitabine received 1000 mg/m 2 as an intravenous infusion over 30-40 minutes weekly for 7 weeks followed by a 1-week rest period in Cycle 1 then as 1000 mg/m 2 on Days 1, 8, and 15 of each subsequent 28-day cycle. Patients in both arms received treatment until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall survival (OS). Additional outcome measures were progression-free survival (PFS) and overall response rate (ORR), both assessed by independent, central, blinded radiological review using RECIST (version 1.0).
In the intent-to-treat (all randomized) population, the median age was 63 years (range 27-88 years) with 42% ≥ 65 years of age; 58% were men; 93% were White and KPS was 90-100 in 60%. Disease characteristics included 46% of patients with 3 or more metastatic sites; 84% of patients had liver metastasis; and the location of the primary pancreatic lesion was in the head of pancreas (43%), body (31%), or tail (25%).
Results for overall survival, progression-free survival, and overall response rate are shown in Table 13.Table 13: Efficacy Results from Randomized Study in Patients with Adenocarcinoma of the Pancreas (ITT Population) Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound) (125 mg/m 2) and gemcitabine (N = 431) Gemcitabine
(N = 430)Overall Survival Number of deaths, n (%) 333 (77) 359 (83) Median Overall Survival (months) 8.5 6.7 95% CI 7.9, 9.5 6.0, 7.2 HR (95% CI) a 0.72 (0.62, 0.83) P-value b <0.0001 Progression-free Survival c Death or progression, n (%) 277 (64) 265 (62) Median Progression-free Survival (months) 5.5 3.7 95% CI 4.5, 5.9 3.6, 4.0 HR (95% CI) a 0.69 (0.58, 0.82) P-value b <0.0001 Overall Response Rate c Confirmed complete or partial overall response, n (%) 99 (23) 31 (7) 95% CI 19.1, 27.2 5.0, 10.1 P-value d <0.0001 CI = confidence interval, HR = hazard ratio of Paclitaxel Protein-bound Particles for Injectable Suspension (albumin-bound) plus gemcitabine / gemcitabine, ITT = intent-to-treat population.
a Stratified Cox proportional hazard model.
b Stratified log-rank test stratified by geographic region (North America versus Others), Karnofsky performance score (70 to 80 versus 90 to 100), and presence of liver metastasis (yes versus no).
c Based on Independent Radiological Reviewer Assessment.
d Chi-square test.
In exploratory analyses conducted in clinically relevant subgroups with a sufficient number of subjects, the treatment effects on overall survival were similar to that observed in the overall study population.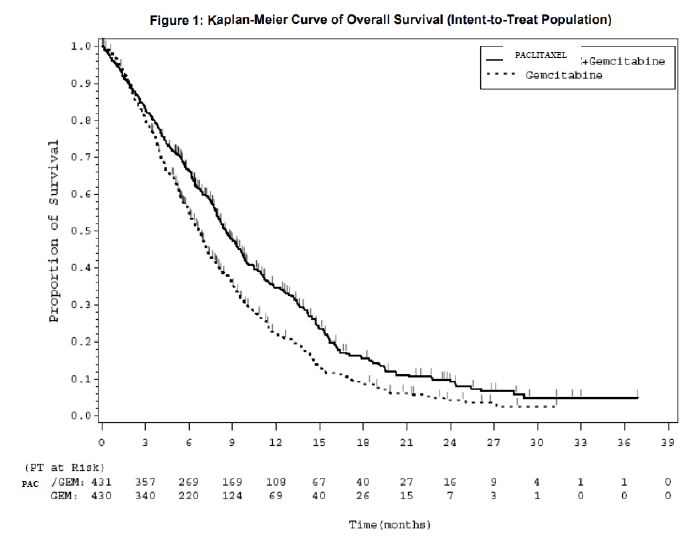
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is a white to yellow, sterile lyophilized powder supplied as:
NDC: 0781-3531-91 100 mg of paclitaxel in a single-dose vial, individually packaged in a carton.
Store the vials in original cartons at 20°C to 25°C (68°F to 77°F). Retain in the original package to protect from bright light.
Discard unused portion.
Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the approved patient labeling (Patient Information).
Severe Myelosuppression
- Patients must be informed of the risk of low blood cell counts and severe and life-threatening infections and instructed to contact their healthcare provider immediately for fever or evidence of infection [see Warnings and Precautions (5.1), (5.3)].
Severe Neuropathy
- Patients must be informed that sensory neuropathy occurs frequently with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and patients should advise their healthcare providers of numbness, tingling, pain, or weakness involving the extremities [see Warnings and Precautions (5.2)].
Pneumonitis
- Instruct patients to contact their healthcare provider immediately for sudden onset of dry persistent cough, or shortness of breath [see Warnings and Precautions (5.4)] .
Severe Hypersensitivity
- Instruct patients to contact their healthcare provider for signs of an allergic reaction, which could be severe and sometimes fatal [see Warnings and Precautions (5.5)].
Common Adverse Reactions
- Explain to patients that alopecia, fatigue/asthenia, and myalgia/arthralgia occur frequently with paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- Instruct patients to contact their healthcare providers for persistent vomiting, diarrhea, or signs of dehydration [see Adverse Reactions (6)] .
Embryo-Fetal Toxicity
- Paclitaxel protein-bound particles for injectable suspension (albumin-bound) injection can cause fetal harm. Advise patients to avoid becoming pregnant while receiving this drug. Females of reproductive potential should use effective contraception during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least six months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Warnings and Precautions (5.8)and Use in Specific Populations (8.1, 8.3)].
- Advise male patients with female partners of reproductive potential to use effective contraception and avoid fathering a child during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for at least three months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) [see Use in Specific Populations (8.3)].
Lactation
- Advise patients not to breastfeed while taking paclitaxel protein-bound particles for injectable suspension (albumin-bound) and for two weeks after receiving the last dose [see Use in Specific Populations (8.2)] .
Infertility
- Advise males and females of reproductive potential that paclitaxel protein-bound particles for injectable suspension (albumin-bound) may impair fertility [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 05/2025 Patient Information Paclitaxel (pak-li-TAX-el) Protein-Bound Particles for Injectable Suspension (Albumin-Bound) What is paclitaxel protein-bound particles for injectable suspension (albumin-bound)? Paclitaxel protein-bound particles for injectable suspension (albumin-bound) is a prescription medicine used to treat: - advanced breast cancer in people who have already received certain other medicines for their cancer.
- advanced non-small cell lung cancer (NSCLC), in combination with carboplatin in people who cannot be treated with surgery or radiation.
- advanced pancreatic cancer, when used in combination with gemcitabine as the first medicine for advanced pancreatic cancer
It is not known if paclitaxel protein-bound particles for injectable suspension (albumin-bound) is safe or effective in children. Do not receive paclitaxel protein-bound particles for injectable suspension (albumin-bound) if: - your white blood cell count is below 1,500 cells/ mm 3.
- you have had a severe allergic reaction to paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Before you receive paclitaxel protein-bound particles for injectable suspension (albumin-bound), tell your healthcare provider about all of your medical conditions, including if you: - have liver or kidney problems.
- had a prior allergic reaction to a taxane.
- are pregnant or plan to become pregnant. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before you start treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- You should not become pregnant during your treatment and for at least six months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- You should use effective birth control (contraception) during your treatment and for at least six months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound). Talk to your healthcare provider about birth control methods you can use during this time.
Males with a female sexual partner who can become pregnant:- Paclitaxel protein-bound particles for injectable suspension (albumin-bound) can harm the unborn baby of your partner.
- You should not father a child during your treatment and for at least three months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- You should use effective birth control (contraception) during your treatment and for at least three months after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- are breastfeeding or plan to breastfeed. Do not breastfeed during your treatment and for two weeks after the last dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list to show your healthcare provider and pharmacist when you get a new medicine. How will I receive paclitaxel protein-bound particles for injectable suspension (albumin-bound)? - Your healthcare provider will prescribe paclitaxel protein-bound particles for injectable suspension (albumin-bound) in an amount that is right for you.
- Your healthcare provider may give you certain medicines to help prevent allergic reactions if you have had an allergic reaction to paclitaxel protein-bound particles for injectable suspension (albumin-bound) in the past.
- Paclitaxel protein-bound particles for injectable suspension (albumin-bound) will be given to you by intravenous (IV) infusion into your vein.
- Your healthcare provider should do blood tests regularly during treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- Your healthcare provider may stop your treatment, delay your treatment, or change your dose of paclitaxel protein-bound particles for injectable suspension (albumin-bound) if you have certain side effects.
What are the possible side effects of paclitaxel protein-bound particles for injectable suspension (albumin-bound)? Paclitaxel protein-bound particles for injectable suspension (albumin-bound) may cause serious side effects, including: - severe decreased blood cell counts. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) can cause a severe decrease in neutrophils, a type of white blood cell which helps fight infections, and blood cells called platelets which help to clot blood. Your healthcare provider will check your blood cell count during your treatment with paclitaxel protein-bound particles for injectable suspension (albumin-bound).
- severe nerve problems (neuropathy).Tell your healthcare provider if you have numbness, tingling, pain, or weakness in your hands or feet.
- severe infection (sepsis).If you receive paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine, infections can be severe and lead to death. Tell your healthcare provider right away if you have a fever (temperature greater than 100.4° F) or develop signs of infection.
- lung or breathing problems.If you receive paclitaxel protein-bound particles for injectable suspension (albumin-bound) in combination with gemcitabine, lung or breathing problems may be severe and can lead to death. Tell your healthcare provider right away if you suddenly get a dry cough that will not go away or shortness of breath.
- severe allergic reactions.Severe allergic reactions are medical emergencies that can happen in people who receive paclitaxel protein-bound particles for injectable suspension (albumin-bound) and can lead to death. You may have an increased risk of having an allergic reaction to paclitaxel protein-bound particles for injectable suspension (albumin-bound) if you are allergic to other taxane medicines. Your healthcare provider will monitor you closely for allergic reactions during your infusion of paclitaxel protein-bound particles for injectable suspension (albumin-bound). Tell your healthcare provider right away if you get any of these signs of a serious allergic reaction: trouble breathing, sudden swelling of your face, lips, tongue, throat, or trouble swallowing, hives (raised bumps), rash, or redness all over your body.
The most common side effects of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in people with breast cancer include: - hair loss
- numbness, tingling, pain, or weakness in the hands or feet
- tiredness
- changes in your liver function tests
- nausea
- diarrhea
- infections
- decreased white blood cell count
- abnormal heartbeat
- joint and muscle pain
- low red blood cell count (anemia). Red blood cells carry oxygen to your body tissues. Tell your healthcare provider if you feel weak, tired, or short of breath.
The most common side effects of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in people with non-small cell lung cancer include: - low red blood cell count (anemia)
- decreased platelet cell count
- numbness, tingling, pain, or weakness in the hands or feet
- tiredness
- decreased white blood cell count
- hair loss
- nausea
The most common side effects of paclitaxel protein-bound particles for injectable suspension (albumin-bound) in people with pancreatic cancer include: - decreased white bolld cell count
- numbness, tingling, pain, or weakness in the hands or feet
- hair loss
- diarrhea
- vomiting
- rash
- tiredness
- nausea
- swelling in the hands or feet
- fever
- decreased appetitie
- signs of dehydration including thirst, dry mouth, dark yellow urine, decreased urine, headache, or muscle cramps
Tell your healthcare provider if you have vomiting, diarrhea, or signs of dehydration that does not go away. Paclitaxel protein-bound particles for injectable suspension (albumin-bound) may cause fertility problems in males and females, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you. These are not all of the possible side effects of paclitaxel protein-bound particles for injectable suspension (albumin-bound).
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of paclitaxel protein-bound particles for injectable suspension (albumin-bound). Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about paclitaxel protein-bound particles for injectable suspension (albumin-bound) that is written for health professionals. What are the ingredients in paclitaxel protein-bound particles for injectable suspension (albumin-bound)? Active ingredient:paclitaxel (bound to human albumin). Other ingredient:human albumin (containing sodium caprylate and sodium acetyltryptophanate). Manufactured by Jiangsu Hengrui Pharmaceuticals Co., Ltd.,
Lianyungang, Jiangsu 222069, China for
Sandoz Inc., Princeton, NJ 08540
For more information, call 1-800-525-8747. 25AUF03
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Vial
NDC: 0781-3531-91
Paclitaxel protein-bound particles for injectable suspension (albumin-bound)

-
INGREDIENTS AND APPEARANCE
PACLITAXEL
paclitaxel injection, powder, lyophilized, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0781-3531 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PACLITAXEL (UNII: P88XT4IS4D) (PACLITAXEL - UNII:P88XT4IS4D) PACLITAXEL 100 mg in 20 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0781-3531-91 1 in 1 CARTON 10/09/2024 1 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212700 10/08/2024 Labeler - Sandoz Inc (005387188)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.