SYMVESS- acellular tissue engineered vessel implant
Symvess by
Drug Labeling and Warnings
Symvess by is a Prescription medication manufactured, distributed, or labeled by Humacyte Global, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SYMVESS safely and effectively. See full prescribing information for SYMVESS.
SYMVESS™ (acellular tissue engineered vessel-tyod), for surgical vascular implantation
Initial U.S. Approval: 2024INDICATIONS AND USAGE
SYMVESS™ is an acellular tissue engineered vessel indicated for use in adults as a vascular conduit for extremity arterial injury when urgent revascularization is needed to avoid imminent limb loss, and autologous vein graft is not feasible. (1)
DOSAGE AND ADMINISTRATION
For surgical vascular implantation only.
- SYMVESS is administered by surgical implantation to replace the injured extremity artery. The anatomical location and length are decided upon at the discretion of the implanting surgeon and based on the appropriate preoperative clinical evaluation, vessel mapping and/or imaging. (2.1)
- SYMVESS may be trimmed to provide the length required for each vascular repair. Additionally, a single SYMVESS can be cut into different lengths and used to repair more than one injured extremity artery in the same patient. Each SYMVESS unit is for administration to a single patient only. (2.1)
DOSAGE FORMS AND STRENGTHS
SYMVESS is an acellular tissue engineered vessel composed of organized extracellular matrix (ECM) proteins. It has dimensions of 6 mm in inner diameter and 42 cm in length (approximately 40 cm of usable length). (3)
CONTRAINDICATIONS
DO NOT use SYMVESS in patients who have a medical condition that would preclude long-term antiplatelet therapy (such as aspirin or clopidogrel) after resolution of acute injuries. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥3%) were thrombosis, fever, pain, anastomotic stenosis, rupture or anastomotic failure, and infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Humacyte Global, Inc. at 1-833-591-0081 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: GRAFT FAILURE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Handling of SYMVESS
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Graft Rupture
5.2 Anastomotic Failure
5.3 Thrombosis
5.4 Transmission of Infectious Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: GRAFT FAILURE
- Loss of SYMVESS integrity due to mid-graft rupture or anastomotic failure can result in life threatening hemorrhage. [See Warnings and Precautions (5.1, 5.2)]
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
SYMVESS is administered by surgical implantation to replace the injured extremity artery. The anatomical location in the extremity and length required should be determined by the surgeon implanting the graft based on the appropriate pre-operative clinical evaluation, vessel mapping and/or imaging, and intra-operative considerations. SYMVESS is provided as follows: 6 mm in internal diameter and 42 cm in length. Once removed from packaging, its usable length is approximately 40 cm.
Each package (i.e., box containing Tyvek®-sealed tray) contains one SYMVESS unit for administration to a single patient only.
SYMVESS may be trimmed to provide the length required for the artery replacement. SYMVESS can be trimmed to replace more than one injured extremity artery in the same patient.
2.2 Handling of SYMVESS
Supplies:
The following supplies are not included in the carton, but may be needed for the handling of SYMVESS:
1) Sterile basin (as needed) – see Step 8 for details
2) Sheath tunneler (as needed) – see Section 2.3 and Step 3 for details
SYMVESS is to be handled in an appropriate surgical environment as follows:
Step 1: SYMVESS box contains a plastic thermoformed tray with a Tyvek® lid. Open the box and remove the plastic tray (Figure 1).
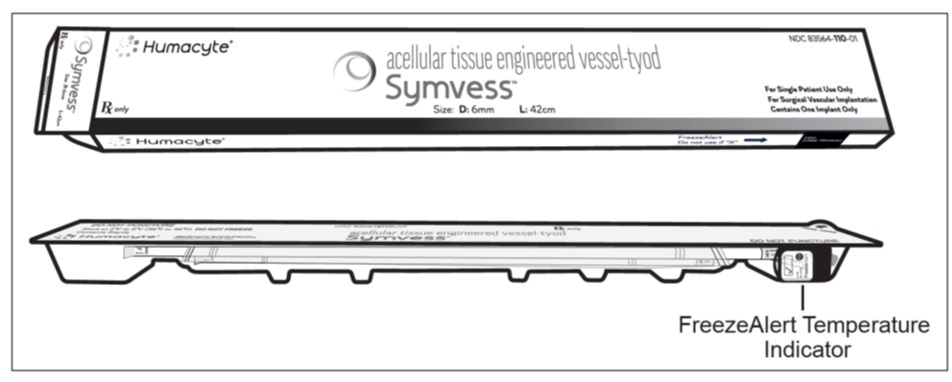
Figure 1: SYMVESS box and plastic trayStep 2: After removing the tray from the box, check the FreezeAlert temperature indicator (Figure 2) attached to the side of the tray. If a check mark is displayed, SYMVESS has not been exposed to freezing temperatures and can be used. If an “X” is displayed, SYMVESS has been exposed to freezing temperatures and should NOT be used.
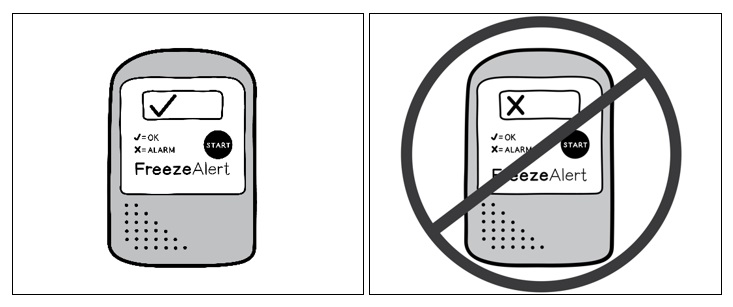
Figure 2: FreezeAlert Temperature indicatorStep 3: Two operators (one sterile operator and one non-sterile operator) are required to remove the sterile packaging bag containing SYMVESS from the plastic tray. Inspect the plastic tray to check for damage which can lead to loss in sterility of the SYMVESS. Do not open the tray for further use if the product is expired or has visible damage. If packaging is intact and within date of acceptable use, the non-sterile operator pulls back the Tyvek® lid by the corner tab to open the plastic tray containing the sterile packaging bag (Figure 3a and 3b). Take careful steps to avoid touching the sterile contents inside the tray (Figure 3c).
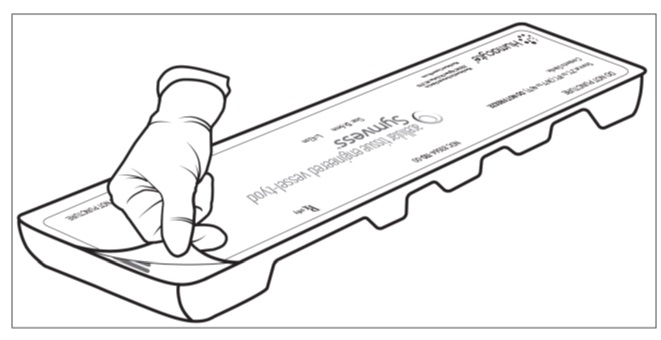
Figure 3a: Non-sterile operator pulls back the Tyvek® lid by the corner tab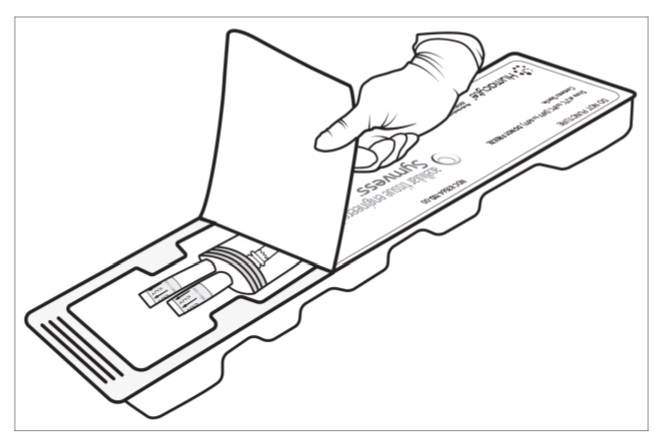
Figure 3b: Non-sterile operator opens the plastic tray containing the sterile packaging bagFigure 3c displays the components of SYMVESS within the plastic tray.
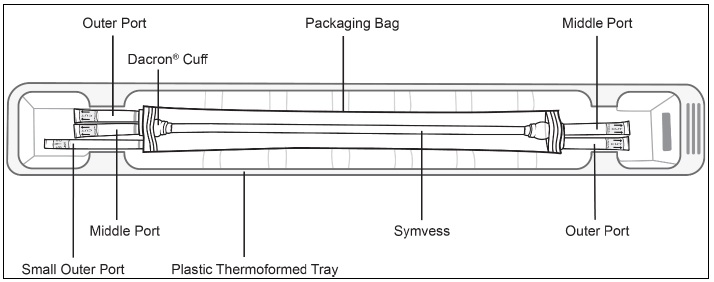
Figure 3c: SYMVESS with each componentStep 4: The non-sterile operator holds the bottom of the plastic tray with one hand and opens the Tyvek® lid with the other. The sterile operator removes the sterile packaging bag from the tray by grasping the ports (i.e., tubing) at the ends of the sterile packaging bag to remove the sterile packaging bag from the tray (Figure 4).
Place the sterile packaging bag on a sterile surface.
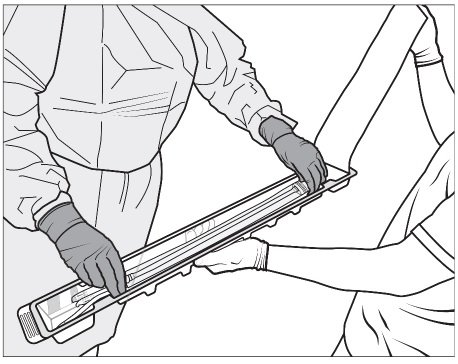
Figure 4: Sterile operator (grey gown and gloves; left) removes the packaging bag from plastic tray by grasping the ports (tubing) while non-sterile operator (white gloves; right) holds the plastic tray and opens the Tyvek® lidStep 5: SYMVESS is suspended in sterile saline inside the packaging. The sterile operator cuts one or both of the outer ports on both ends of the packaging using sterile surgical scissors and drains the sterile saline solution contained within the packaging bag (Figure 5a, 5b and 5c).
NOTE: If the middle ports or the small outer port are accidentally cut, there is no need for concern.
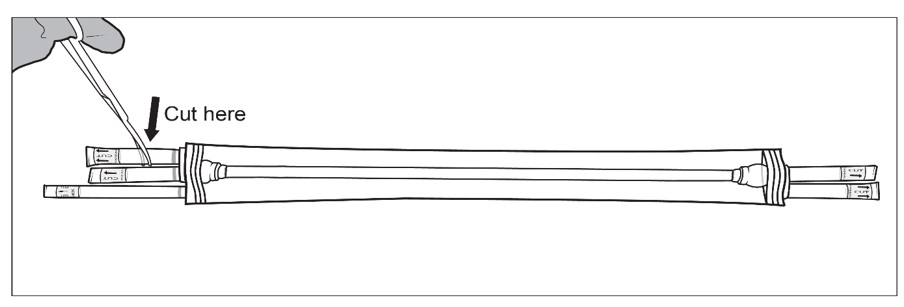
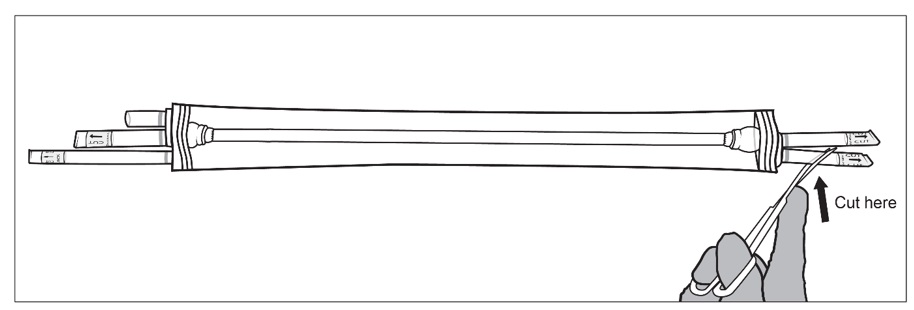
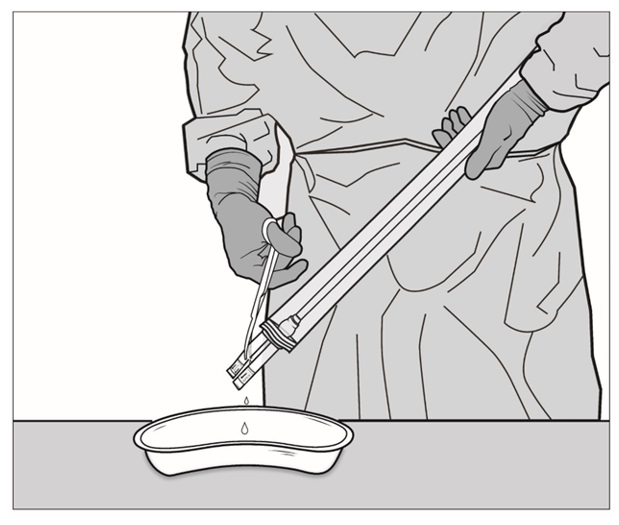
Figure 5a, 5b and 5c: Sterile operator cuts both outer ports one at a time to drain saline solution using surgical scissorsStep 6: Once the saline solution has been drained, place the packaging bag back on a sterile surface. Using sterile surgical scissors or scalpel blade, cut a small slit on the edge of the packaging bag (Figure 6a), then make a second cut along the length of the packaging bag (Figure 6b). Be careful not to cut SYMVESS. If SYMVESS is accidentally nicked or cut during removal from the packaging bag, discard the damaged SYMVESS, and use a new SYMVESS. Make another cut on the other end of the packaging bag to allow for full exposure of SYMVESS (Figure 6c).
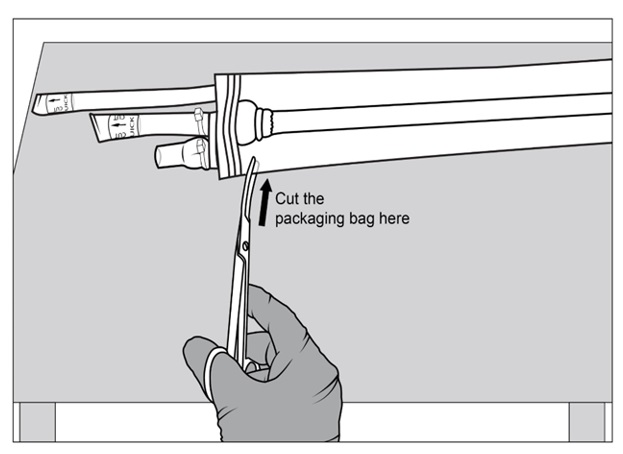
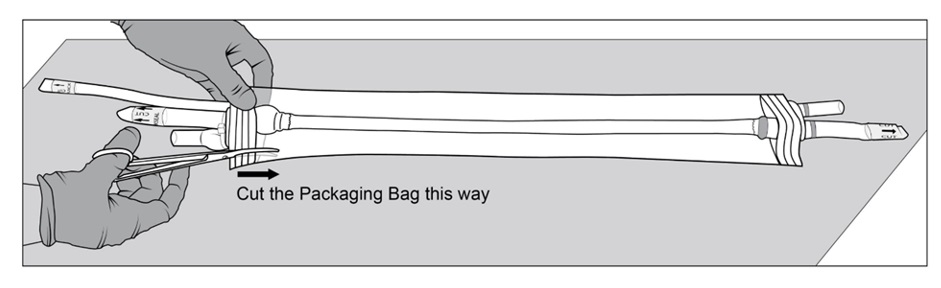
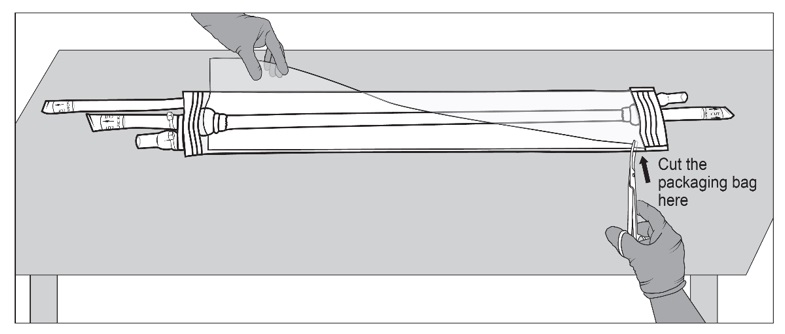
Figure 6a, 6b and 6c: Sterile operator cuts the packaging bag using surgical scissors to reveal SYMVESSStep 7: Holding back the flaps of the open packaging bag, cut SYMVESS 1 cm from the Dacron® cuffs (below the blue sutures) to free it from the packaging bag (Figure 7). Approximately 40 cm in length of SYMVESS should be available for use.
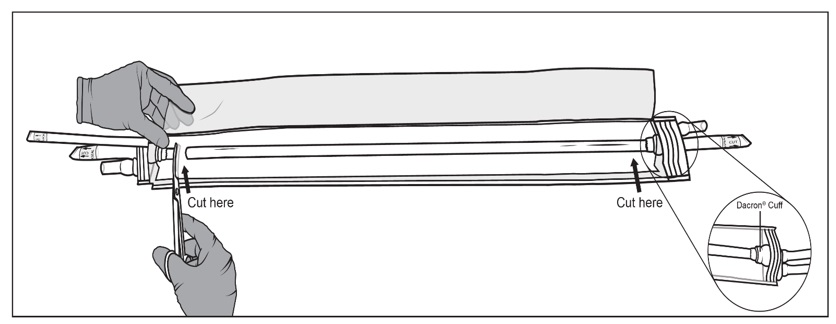
Figure 7: Sterile operator cuts SYMVESS 1 cm from the Dacron® cuff using surgical scissorsStep 8: Use sterile gloves or atraumatic instruments when handling SYMVESS. SYMVESS is ready for immediate implantation OR should be kept immersed in a basin of sterile saline solution at room temperature while the patient is being prepared for surgical implantation (Figure 8). SYMVESS may be immersed in sterile saline solution for up to eight hours during surgical preparation for implantation. IF SYMVESS DRIES OUT, IT IS NOT USABLE AND MUST BE DISCARDED.
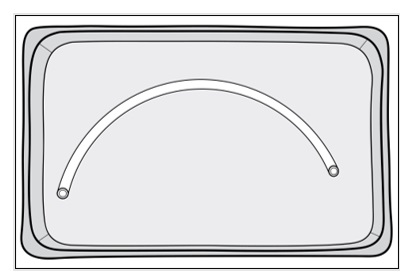
Figure 8: Store SYMVESS in a basin of sterile saline solutionStep 9: SYMVESS contains a clear silicone tube within the lumen. The silicone tube provides structural support to prevent kinking and twisting of SYMVESS when tunneling. The silicone tube can be left in place during any tunneling procedure but should be removed before making an anastomosis. To remove the inner silicone tube, gently grasp the end of SYMVESS and slide SYMVESS down to reveal the inner silicone tube, which can then be pulled out (Figure 9).
2.3 Administration
Surgically implant SYMVESS under appropriate aseptic conditions.
Follow the steps below for implantation of SYMVESS:
- When handling SYMVESS, avoid using excessive force, which may lead to SYMVESS damage.
- To avoid damage or contamination, always use sterile gloves and atraumatic instruments when handling SYMVESS. Always protect SYMVESS from damage by heavy or sharp instruments.
- When necessary, a SHEATH TUNNELER may be used to safely deliver SYMVESS through subcutaneous or other tissues. Create a tissue tunnel that closely matches SYMVESS diameter (6 mm tunneller is recommended, but 8 mm tunneller is acceptable). A KELLY-WICK OR NON-SHEATHED TUNNELLER IS NOT RECOMMENDED FOR USE WITH SYMVESS. During the tunneling process, ensure that SYMVESS does not kink or twist within the tunnel. A twisted or kinked vessel will result in compromised flow and performance, thereby increasing the risk of thrombosis and failure.
- It is recommended to keep the inner silicone tube in place within SYMVESS until any tunneling process is complete. Lubricate the lumen of the tunneller sheath with saline solution and then gently push SYMVESS with the silicone tube through the sheath (alternatively SYMVESS can be grasped by or secured to the inner sheath rod and pulled through the sheath). After the tunneling sheath is removed, the inner silicone tube is removed from SYMVESS.
THE INNER SILICONE TUBE MUST BE REMOVED BEFORE CREATING AN ANASTOMOSIS (FIGURE 9)
Figure 9: Remove the inner silicone tube within the lumen of SYMVESS BEFORE creating an anastomosis.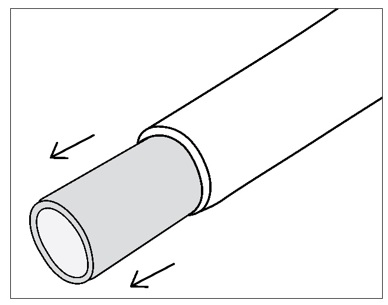
- Following standard surgical procedures, the appropriate length to use for SYMVESS must be carefully determined. SYMVESS DOES NOT ELONGATE UNDER PRESSURE. Since SYMVESS does not elongate, the length must allow SYMVESS to adequately reach both anastomoses. SYMVESS should either be tunneled or held at the site of implantation so that the length to adequately reach both anastomoses is clear. The patient’s body size and posture, and the range of the motions likely to be encountered around the anatomical region of the implantation should be considered when determining length. Choosing the appropriate length of SYMVESS may prevent kinking, twisting, or damaging SYMVESS after implantation.
- Failure to carefully cut SYMVESS may cause damage and result in failure at the anastomosis site or reduced suture retention strength. Cut SYMVESS with sharp vascular scissors as one would trim a human blood vessel prior to implantation. SYMVESS can be held with atraumatic instruments or sterile gloves while cutting with vascular scissors.
- The anastomosis must be precise, and with meticulous attention to detail. SYMVESS may be anastomosed end-to-end or end-to-side with the patient’s vessels. An end-to-side anastomosis may accommodate size discrepancies between the host vessel and SYMVESS. Use of an appropriate anastomotic angle may minimize stresses upon SYMVESS which may reduce kinking and/or mechanical disruptions of SYMVESS, recipient or donor vessel, and/or suture lines.
- Use of non-crushing vascular (atraumatic) clamp is recommended on SYMVESS to avoid mechanical damage or disruption to SYMVESS. AVOID REPEATED, LOCALIZED CLAMPING OR EXCESSIVE CLAMPING ON ANY SECTION OF SYMVESS.
- Use only 5-0 or 6-0 non-absorbable, monofilament suture with size selected based on the nature of the anastomosis. Use of a suture size larger in diameter than 5-0 or smaller in diameter than 6-0 for anastomosis creation with SYMVESS may reduce suture retention.
- A suture bite depth of 2.0-2.5mm is recommended to ensure suture retention.
- Flush static blood from the lumen of SYMVESS while completing the second anastomosis. Failure to do so may result in thrombosis of the vessel.
- Undue anastomotic bleeding may occur if gaps are present between SYMVESS and the host vessel. Use appropriate suture placement and avoid undue tension on the suture line. Topical hemostatic agents may be used to minimize anastomotic bleeding if desired, therefore, the use of these agents will be at the surgeon’s discretion.
- Inspect the operative site carefully prior to closure. Ensure that SYMVESS is covered by healthy muscle and/or soft tissue. Ensure that there are no twists, kinks, redundancy, before and during the tissue closure portion of the case. It may be necessary to clear or release tissue to avoid SYMVESS impingement.
- At the conclusion of the operation, inspect the circulation to ensure that perfusion to the distal vascular bed has not been compromised and assess for risk of compartment syndrome following revascularization.
- Surgical interventions are permissible and up to the surgeon’s discretion. PERCUTANEOUS INTERVENTIONS SHOULD ONLY USE BALLOONS THAT DO NOT EXCEED 6MM IN DIAMETER. Larger diameter balloons used for angioplasty or thrombectomy procedures may result in tearing of the SYMVESS wall or development of pseudoaneurysm. Mechanical thrombectomy devices other than balloons have not been studied with SYMVESS in the trauma setting and therefore are not recommended.
-
3 DOSAGE FORMS AND STRENGTHS
SYMVESS is an acellular tissue engineered vessel composed of organized extracellular matrix (ECM) proteins. SYMVESS is implanted using standard surgical techniques as a vascular construct to replace a patient’s damaged extremity artery after injury. SYMVESS has dimensions of 6 mm in inner diameter and 42 cm in length (approximately 40 cm of usable length). Since the length of SYMVESS to be implanted is dependent on the surgical needs, it is cut to length by the surgeon in the operating room [see Administration (2.3 steps 5 and 6)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Graft Rupture
Vascular graft rupture has occurred in patients treated with SYMVESS [see Clinical Trials Experience (6.1)]. Advise patients that arterial bleeding can be life-threatening and to seek emergent medical evaluation for any signs or symptoms of graft rupture such as bleeding, pain and swelling in the extremity, or signs of extremity ischemia.
Follow appropriate procedures for handling and administering SYMVESS [see Dosage and Administration (2)].
5.2 Anastomotic Failure
Anastomotic failure has occurred in patients treated with SYMVESS [see Clinical Trials Experience (6.1)]. In clinical studies of SYMVESS, anastomotic failure occurred within the first 36 days post-implantation.
Monitor patients for signs of anastomotic failure such as pain and swelling at the surgical site, decreasing hemoglobin or other signs and symptoms of bleeding. Advise patients to seek urgent medical evaluation if they have any signs or symptoms that may be indicative of anastomotic failure such as bleeding, swelling or worsening pain at the surgical site or changes in color of overlying skin.
Follow appropriate procedures for handling and administering SYMVESS [see Dosage and Administration (2)].
5.3 Thrombosis
Thrombosis has occurred in patients treated with SYMVESS [see Clinical Trials Experience (6.1)]. In clinical trials of SYMVESS, patients received antiplatelet therapy following implantation of SYMVESS to reduce the risk of thrombosis. The risk of thrombosis may increase in patients who discontinue antiplatelet therapy.
Anti-platelet therapy is recommended following treatment with SYMVESS.
5.4 Transmission of Infectious Diseases
SYMVESS is manufactured using cells and reagents that may transmit infectious diseases or infectious agents. The cells used in the manufacture of SYMVESS are derived from a donor who met the donor eligibility requirements for transmissible infectious diseases which includes screening and testing of risks associated with human immunodeficiency virus 1 (HIV-1), human immunodeficiency virus 2 (HIV-2), hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis (Treponema pallidum). The cell banks are tested negative for human and animal viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma. While all animal-derived reagents are tested for animal viruses, bacteria, fungi, and mycoplasma before use, these measures do not eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents. Fetal bovine serum is sourced to minimize the risk of transmitting a prion protein that causes bovine spongiform encephalopathy and the cause of a rare fatal condition in humans called variant Creutzfeldt-Jakob disease. No transmissible agent infections have been reported during clinical testing.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to adverse reaction rates in clinical trials of other products, and they may not reflect the rates observed in real-world clinical practice.
The safety data described in this section reflect exposure to SYMVESS in one single arm, open-label study in patients requiring vascular replacement or reconstruction for life or limb threatening vascular trauma, Study 1 (CLN-PRO-V005; NCT03005418). A total of 54 adults received extremity implantation of SYMVESS as a vascular conduit.
The patient population ranged in age from 18 to 72 years (mean age 33 years). Each patient received a SYMVESS implant ranging in length from 1 to 35 cm (mean length 10.1 cm). The most frequently occurring adverse reactions are shown in Table 1.
Table 1 Adverse Reactions Occurring with a Frequency of ≥3% in Study 1 Adverse Reaction* Extremity Patients (%)†
(n=54)- * Adverse Reaction Frequency based on up to 3 years follow-up data [median 191 days (range 1-1134 days)]
- † Extremity patients are with arterial repair in upper or lower limbs
Vascular graft thrombosis 15 (28%) Pyrexia (fever) 9 (17%) Pain 8 (15%) Anastomotic stenosis 5 (9%) Vascular graft rupture or anastomotic failure 4 (7%) Vascular graft infection 3 (6%) Long term follow-up of up to 36 months in Study 1 is ongoing. At the data cut-off date, 7 out of 54 patients in the extremity group have completed the study (i.e., completed 36 months of follow-up).
The safety of SYMVESS beyond 36 months was not evaluated in the clinical studies for this indication.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited clinical data that are available with SYMVESS use in pregnant women are insufficient to inform a drug-associated risk. No animal reproductive and developmental toxicity studies have been conducted with SYMVESS to assess whether it can cause harm to the mother or fetus when administered to a pregnant woman.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the transmission of antibodies or any components of SYMVESS in human milk, the effects on the breast-fed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SYMVESS and any potential adverse effects on the breastfed infant from SYMVESS or from the underlying maternal condition.
-
11 DESCRIPTION
SYMVESS is a sterile, acellular tissue engineered vessel composed of human extracellular matrix (ECM) proteins typically found in human blood vessels. SYMVESS is 6 mm in internal diameter and 42 cm in length (approximately 40 cm of usable length).
SYMVESS is manufactured using a tissue engineering process. Human vascular smooth muscle cells that are derived from human aortic tissue deemed suitable for transplant are banked, expanded, and seeded onto a tubular mesh scaffold. The cell-seeded scaffold is cultured in a biomimetic bioreactor system to generate an intermediate tubular construct containing vascular smooth muscle cells and the extracellular matrix the cells deposited. A final decellularization process removes the human cellular and genetic material while maintaining the extracellular matrix structure and mechanical and biological activity.
SYMVESS manufacture includes use of reagents derived from human and animal materials. Human male AB serum and fetal bovine serum (FBS) are used in the manufacturing process for the cultivation and expansion of the vascular smooth muscle cells.
SYMVESS is fixed inside the packaging bag between the endcaps with a supporting silicone tube and is immersed in sterile phosphate buffered saline solution.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SYMVESS is an acellular tissue engineered vessel composed of human extracellular matrix (ECM) that exhibits mechanical and biological activity. Product testing results have shown that SYMVESS can withstand the forces associated with the arterial blood flow and suturing during implantation. Product testing results have also demonstrated that SYMVESS ECM can support cell binding and proliferation. However, the exact mechanism of action has not been established.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Conventional carcinogenicity and reproductive and developmental toxicity studies have not been conducted. Results from in vitro studies including a bacterial reverse mutation assay, L-929 mouse fibroblast assay, and human lymphocyte chromosomal aberration assay demonstrated that SYMVESS material is non-mutagenic.
-
14 CLINICAL STUDIES
The efficacy and safety of SYMVESS as a vascular conduit for extremity arterial trauma repair was evaluated in a subset of patients enrolled in Study 1 (CLN-PRO-V005; NCT03005418) who had extremity arterial injuries that were life or limb threatening, and in Study 2 (CLN-PRO-V017; NCT05873959) as described below.
Study 1
This is a prospective, single arm, multicenter study conducted to evaluate safety and efficacy of SYMVESS for vascular replacement in patients with life or limb threatening vascular trauma. For inclusion in the study, patients must have been adults with an arterial defect length less than or equal to 38 cm that must be appropriately size matched to the 6 mm SYMVESS inner diameter. The study excluded patients with a mangled extremity severity score (MESS) greater than or equal to 7 or an abbreviated injury scale (AIS) greater than 5 from study participation.
The primary efficacy outcome measure was the rate of primary vascular graft patency at Day 30 in patients with extremity arterial injury assessed using duplex ultrasound or equivalent (i.e., CTA, MRI). Additional efficacy outcome measures included secondary patency at Day 30, conduit infection within first 30 days and limb salvage at Day 30 post implantation.
A total of 54 patients with a median age of 30 years (range 18 to 72 years) received SYMVESS as a conduit for extremity arterial trauma repair. Twenty-six patients (48%) were Black or African American and 23 patients (43%) were White and five patients (9%) were classified as other. Forty patients (74%) were male. The distribution of type of trauma was 31 (57%) penetrating trauma and 23 (43%) blunt trauma. The Day 30 efficacy outcomes are summarized in Table 2.
Table 2 Outcomes for the extremity group of Study 1 at Day 30 (N = 54)* Endpoint n=54 % (95% CI) - * Nine patients not available for Day 30 assessment were imputed as failures for patency and limb salvage estimation
Primary Patency 36 66.7% (53.4%, 77.8%) Secondary Patency 39 72.2% (59.1%, 82.4%) Vascular Graft Infections 1 1.9% (0.3%, 9.8%) Limb Salvage 41 75.9% (63.1%, 85.4%) Nine (9) patients had confirmed loss of primary patency over the first thirty days, of which, six patients had also lost secondary patency. Of the thirteen patients not available for Day 30 evaluation, four patients had confirmed loss of patency (one SYMVESS infection and removal and subsequent amputation, three patients with thrombosis leading to SYMVESS removal in two patients and the thrombosed SYMVESS left in place with good collateral circulation in the third patient). The remaining nine patients who were not evaluable for patency assessment were due to loss to follow-up (1 patient), amputation (4 patients), or death (4 patients).
Seven (7) out of 54 (13%) patients completed 36-month follow-up with a further 10 (19%) patients ongoing at the time of data cutoff for the 120-day safety report. Reasons for early discontinuation from the study included: loss to follow-up (n=13, 24%), SYMVESS abandonment (n=10, 19%), death (n=6, 11%), limb amputation (n=6, 11%), and withdrawal of consent (n=2, 4%). Patient follow-up ranged from 1-1134 days (median = 191 days) with 41 (76%) patients who were followed for at least 30 days, 30 (56%) patients were followed for at least six months and 17 (32%) patients were followed for at least one year.
Study 2
This is an observational study consisting of a retrospective analysis of point of care clinical data in patients who received SYMVESS in Ukraine. The study included patients who received SYMVESS to repair traumatic life- or limb-threatening vascular extremity injuries. Patients with a damaged upper or lower limb artery less than or equal to 38 cm and appropriately size matched to the 6 mm SYMVESS inner diameter were included. The study excluded patients with a mangled extremity severity score (MESS) of > 7 or an abbreviated injury scale (AIS) > 5.
The primary efficacy outcome measure was the rate of primary vascular graft patency at Day 30 using duplex ultrasound. Additional efficacy outcome measures included secondary patency at Day 30, conduit infection within first 30 days and limb salvage at Day 30 post implantation. Among the 16 patients in the study, the median age was 31 years (range 22 to 54 years). All patients were White and male. The distribution of type of trauma was 87.5% penetrating and 12.5% blunt trauma. The median follow-up duration in Study 2 was 366 days (range 13-573 days). SYMVESS implanted length ranged from 3.5 to 40 cm (mean = 18.3 cm). All 16 (100%) patients were evaluable for 30-day patency. Fifteen patients (94%) retained primary and secondary patency at Day 30. One (1) patient had SYMVESS replaced on Day 13 due to recurrent bleeding following rupture of conduit. Infection rate was 0% at Day 30. Limb salvage rate was 100% at Day 30.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SYMVESS is for single patient use only and is supplied in a sealed sterile packaging bag. The packaging bag is contained within a plastic thermoformed tray sealed with a Tyvek® lid, the contents of which are sterile. The sealed plastic thermoformed tray (NDC: 83564-110-00) is contained within a non-sterile carton (NDC: 83564-110-01).
SYMVESS is fixed inside the packaging bag between the endcaps with a supporting silicone tube and is immersed in sterile phosphate buffered saline solution. SYMVESS, the saline solution, and the outer surface of the packaging bag are sterile.
SYMVESS is shipped in an insulated shipping box (2ºC to 8ºC).
16.2 Storage and Handling
Store SYMVESS at 2ºC to 8ºC (36ºF to 46ºF).
Use SYMVESS prior to the expiration date printed on the carton.
Do not freeze.A FreezeAlert temperature indicator is attached to the exterior of each thermoformed tray that contains a SYMVESS. Prior to preparing SYMVESS for a procedure, check the FreezeAlert indicator. A check mark indicates SYMVESS has not been exposed to freezing temperatures and can be used. An “X” displayed on the indicator signifies that SYMVESS has been exposed to freezing temperatures and should NOT be used [see Handling of SYMVESS (2.2)].
DO NOT use SYMVESS if:
- carton has been damaged
- Tyvek® lid is punctured or detached
- thermoformed tray is punctured or compromised
- there is evidence of fluid leakage from the packaging bag
- FreezeAlert temperature indicator has an “X”
Dispose of unused SYMVESS and packaging bag using established clinical facility procedures in accordance with local regulatory requirements.
Contact Customer Care at 1-833-591-0081 for further instructions or questions.
-
17 PATIENT COUNSELING INFORMATION
Counsel the patient on the following:
-
Graft Rupture
Inform patients that bleeding can occur from the graft site and that subsequent intervention, explant, or additional procedures may be required after initial repair. Advise patients to seek urgent medical evaluation if they develop any bleeding from the surgical site, or have any signs or symptoms that would indicate failure of the graft such as worsening pain, loss of limb function or change in color of overlying skin. [see Warnings and Precautions (5.1)]. -
Anastomotic Failure
Inform patients that in certain situations, bleeding can occur at the graft anastomosis site and that subsequent intervention, explant, or additional procedures may be required to repair this. Advise patients to seek urgent medical evaluation if they have any signs or symptoms that may be indicative of anastomotic failure such as bleeding, swelling or worsening pain at the surgical site or changes in color of overlying skin. [see Warnings and Precautions (5.2)]. -
Thrombosis
Inform patients that in certain situations, thrombosis can occur after SYMVESS implantation. At the surgeon's discretion, consider use of antiplatelet therapy following implantation of SYMVESS to reduce the risk of thrombosis. The risk of thrombosis may increase in patients who discontinue antiplatelet therapy. Advise patients to seek urgent medical evaluation if they have any signs or symptoms that may be indicative of thrombosis including worsening pain at the surgical site, swelling, changes of color of the overlying skin, or warm skin around the painful area. [see Warnings and Precautions (5.3)]. -
Transmission of Infectious Diseases
Inform patients of the possible risk of transmission of infectious diseases with SYMVESS. [see Warnings and Precautions (5.4)].
Manufactured by:
Humacyte Global, Inc.
2525 E. NC Highway 54
Durham, NC 27713
USA
SYMVESS™ is a trademark of Humacyte Global, Inc.
-
Graft Rupture
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
DO NOT PUNCTURE
NDC 83564-110-00
Rx only
PEEL HERE
Store at 2ºC to 8ºC (36ºF to 46ºF). DO NOT FREEZE.
Contents Sterile.
acellular tissue engineered vessel-tyod
Symvess™
Size: D: 6mm L: 42cmHumacyte®
Manufactured by: Humacyte Global, Inc.
2525 NC Highway 54, Durham, NC 27713U.S. License No. 2336
DO NOT PUNCTURE

-
INGREDIENTS AND APPEARANCE
SYMVESS
acellular tissue engineered vessel implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83564-110 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACELLULAR TISSUE ENGINEERED VESSEL (UNII: 6H248RS8YQ) (ACELLULAR TISSUE ENGINEERED VESSEL - UNII:6H248RS8YQ) ACELLULAR TISSUE ENGINEERED VESSEL 420 mm Inactive Ingredients Ingredient Name Strength DPBS 1X, NO CALCIUM, NO MAGNESIUM (UNII: D9FS8LTC4V) Product Characteristics Color Score Shape ROUND Size 6mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83564-110-00 1 in 1 BAG 12/19/2024 1 NDC: 83564-110-01 1 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125812 12/19/2024 Labeler - Humacyte Global, Inc. (557190449) Establishment Name Address ID/FEI Business Operations Humacyte Global, Inc. 557190449 manufacture(83564-110) , analysis(83564-110) , label(83564-110)
Trademark Results [Symvess]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SYMVESS 98050185 not registered Live/Pending |
Humacyte Global, Inc. 2023-06-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
