Ascorbic Acid by Fresenius Kabi USA, LLC ASCORBIC ACID injection
Ascorbic Acid by
Drug Labeling and Warnings
Ascorbic Acid by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ASCORBIC ACID INJECTION safely and effectively. See full prescribing information for ASCORBIC ACID INJECTION.
ASCORBIC ACID injection, for intravenous use
Initial U.S. Approval: 1947INDICATIONS AND USAGE
Ascorbic acid injection is vitamin C indicated for the short term (up to 1 week) treatment of scurvy in adult and pediatric patients age 5 months and older for whom oral administration is not possible, insufficient or contraindicated. (1)
Limitations of Use (1)
Ascorbic acid injection is not indicated for treatment of vitamin C deficiency that is not associated with signs and symptoms of scurvy. (1)
DOSAGE AND ADMINISTRATION
- Supplied in a Pharmacy Bulk Package (PBP). Dispense single doses to multiple patients in a pharmacy admixture program; use within 4 hours of puncture. (2.1)
- Must be diluted prior to use (2.1)
- Administer as a slow intravenous infusion (2.1)
- See Full Prescribing Information for important administration instructions (2.1)
- Maximum recommended duration is one week (2.2)
Population (2.2)
Recommended Doses
Pediatric patients age 5 months to less than 12 months
50 mg once daily
Pediatric patients age 1 year to less than 11 years
100 mg once daily
Adults and pediatric patients age 11 years and older
200 mg once daily
Pregnant women, lactating women, patients with glucose-6-phosphate dehydrogenase deficiency
Should not exceed the U.S. Recommended Dietary Allowance (RDA)
DOSAGE FORMS AND STRENGTHS
Injection: 25,000 mg per 50 mL (500 mg per mL) - Pharmacy Bulk Package (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Oxalate nephropathy and Nephrolithiasis: Ascorbic acid has been associated with development of acute or chronic oxalate nephropathy following prolonged use of high doses of ascorbic acid infusion. Patients with renal disease including renal impairment, history of oxalate kidney stones, geriatric patients, and pediatric patients less than 2 years old may be at increased risk (5.1).
- Hemolysis: Patients with glucose-6-phosphate dehydrogenase deficiency are at risk of severe hemolysis; a reduced dose is recommended (5.2).
- Laboratory Test Interference: Ascorbic acid may interfere with laboratory tests based on oxidation-reduction reactions, including blood and urine glucose testing (5.3).
ADVERSE REACTIONS
Most common adverse reactions are pain and swelling at the site of infusion (6)
To report SUSPECTED ADVERSE REACTIONS, contact [Fresenius Kabi USA, LLC at 1-800-551-7176] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Antibiotics: Ascorbic acid may decrease the activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin. Bleomycin is inactivated in vitro by ascorbic acid (7.1).
- Amphetamine and Other Drugs Affected by Urine Acidification: Ascorbic acid may cause acidification of the urine and result in decreased amphetamine serum levels and affect excretion and plasma concentrations of other drugs sensitive to urine pH (7.2).
- Warfarin: Continue standard monitoring (7.3).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Recommended Dosage
2.3 Dosage Reductions in Specific Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Oxalate Nephropathy and Nephrolithiasis
5.2 Hemolysis in Patients with Glucose-6-Phosphate Dehydrogenase Deficiency
5.3 Laboratory Test Interference
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Antibiotics
7.2 Amphetamines & Other Drugs Affected by Urine Acidification
7.3 Warfarin
7.4 Laboratory Test Interference
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
- Ascorbic acid injection is indicated for the short term (up to 1 week) treatment of scurvy in adult and pediatric patients, age 5 months and older, for whom oral administration is not possible, insufficient or contraindicated.
- Limitations of Use
- Ascorbic acid injection is not indicated for the treatment of vitamin C deficiency that is not associated with signs and symptoms of scurvy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Ascorbic acid injection vials contain 25,000 mg of ascorbic acid and the largest recommended single dose is 200 mg. Do not give the entire contents of the vial to a single patient.
- Do not administer ascorbic acid injection as an undiluted intravenous injection.
- Minimize exposure to light because ascorbic acid injection is light sensitive.
- Ascorbic acid injection is supplied as a Pharmacy Bulk Package (PBP) which is intended for dispensing of single doses to multiple patients in a pharmacy admixture program and is restricted to the preparation of admixtures for infusion:
- a. Use only in a suitable ISO Class 5 work area such as a laminar flow hood (or an equivalent clean air compounding area).
- b. Penetrate each PBP vial closure only one time with a suitable sterile transfer device or dispensing set that allows measured dispensing of the contents. Given that pressure may develop within the vial during storage, exercise caution when withdrawing contents from the vial.
- c. Once the closure system has been penetrated, complete all dispensing from the PBP vial within 4 hours. Each dose must be used immediately. Discard unused portion.
- d. Prior to administration, ascorbic acid injection must be diluted in a suitable infusion solution and the final solution for infusion must be isotonic (undiluted the osmolarity of ascorbic acid injection is approximately 5,900 mOsmol/L). Prior to preparing the admixture for infusion, calculate the osmolarity of the intended admixture for infusion. Add one daily dose of ascorbic acid injection directly to an appropriate volume of a suitable infusion solution (e.g., 5% Dextrose Injection, Sterile Water for Injection) and add appropriate solutes, as necessary, to make the final solution isotonic. Sterile Water for Injection is highly hypotonic; adjust solute content, as necessary, to make the final infusion solution isotonic prior to injection. Do not mix ascorbic acid injection with solutions containing elemental compounds that can be reduced (e.g., copper). The concentration of ascorbic acid in the final, admixture solution for infusion is to be in the range of 1 to 25 mg of ascorbic acid per mL. For example, for the largest recommended dose:
- Add 200 mg of ascorbic acid (equivalent to 0.4 mL of ascorbic acid injection) to 7.5 mL of Sterile Water for Injection to produce an infusion solution having an approximate osmolarity of 290 mOsmol/L. In this specific example, addition of solute is NOT necessary because the solution is isotonic.
- e. Prepare the recommended dose based on the patient population[see Dosage and Administration (2.2),(2.3)].
- f. Visually inspect for particulate matter and discoloration prior to administration (the diluted ascorbic acid injection solution should appear colorless to pale yellow).
- g. Immediately administer the admixture for infusion as a slow intravenous infusion [see Recommended Dosage (2.2)]
2.2 Recommended Dosage
Table 1 provides recommended doses of ascorbic acid injection based on patient population and infusion rates of diluted ascorbic acid injection solution.
Table 1: Recommended Dose of ascorbic acid injection and Infusion Rate of Diluted ascorbic acid injection Solution
Patient Population
Ascorbic acid injection
Once Daily Dose (mg)
Infusion Rate of Diluted Ascorbic acid injection Solution (mg/minute)
Pediatric Patients age 5 months to less than 12 months
50
1.3
Pediatric Patients age 1 year to less than 11 years
100
3.3
Adults and Pediatric Patients 11 years and older
200
33
The recommended maximum duration of daily treatment with ascorbic acid injection is seven days. If no improvement in scorbutic symptoms is observed after one week of treatment, retreat until resolution of scorbutic symptoms is observed.
Repeat dosing is not recommended in pediatric patients less than 11 years of age.
2.3 Dosage Reductions in Specific Populations
Women who are pregnant or lactating and patients with glucose-6-dehydrogenase deficiency should not exceed the U.S. Recommended Dietary Allowance (RDA) or daily Adequate Intake (AI) level for ascorbic acid for their age group and condition [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1,8.2)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Oxalate Nephropathy and Nephrolithiasis
Acute and chronic oxalate nephropathy have been reported with prolonged administration of high doses of ascorbic acid. Acidification of the urine by ascorbic acid may cause precipitation of cysteine, urate or oxalate stones. Patients with renal disease including renal impairment, history of oxalate kidney stones, and geriatric patients may be at increased risk for oxalate nephropathy while receiving treatment with ascorbic acid. Pediatric patients less than 2 years of age may be at increased risk for oxalate nephropathy during treatment with ascorbic acid because their kidneys are immature [see Use in Specific Populations (8.4,8.5,8.6)]. Monitor renal function in patients at increased risk receiving ascorbic Acid Injection. Discontinue ascorbic acid injection in patients who develop oxalate nephropathy and treat any suspected oxalate nephropathy.
Ascorbic acid injection is not indicated for prolonged administration (the maximum recommended duration is one week) [see Dosage and Administration (2.1)].
5.2 Hemolysis in Patients with Glucose-6-Phosphate Dehydrogenase Deficiency
Hemolysis has been reported with administration of ascorbic acid in patients with glucose-6-phosphate dehydrogenase deficiency. Patients with glucose-6-phosphate dehydrogenase deficiency may be at increased risk for severe hemolysis during treatment with ascorbic acid. Monitor hemoglobin and blood count and use a reduced dose of Ascorbic acid injection in patients with glucose-6-phosphate dehydrogenase deficiency [see Dosage and Administration (2.3)]. Discontinue treatment with ascorbic acid injection if hemolysis is suspected and treat as needed.
5.3 Laboratory Test Interference
Ascorbic acid may interfere with laboratory tests based on oxidation-reduction reactions, including blood and urine glucose testing, nitrite and bilirubin levels, and leucocyte count testing. If possible, laboratory tests based on oxidation-reduction reactions should be delayed until 24 hours after infusion of ascorbic acid injection [see Drug Interactions (7.4)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Oxalate nephropathy and Nephrolithiasis [see Warnings and Precautions (5.1)].
- Hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency [see Warnings and Precautions(5.2)].
- The following adverse reactions associated with the use of ascorbic acid were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure:
Administration site reactions: pain and swelling.
Ascorbic acid injection should not be rapidly administered. Rapid intravenous administration (>250 mg/minute) of ascorbic acid injection may cause temporary faintness or nausea, lethargy, flushing, dizziness, and headache (the recommended infusion rates of diluted ascorbic acid injection solution are 1.3 mg/minute (Pediatric Patients age 5 months to less than 12 months), 3.3 mg/minute (Pediatric Patients age 1 year to less than 11 years) and 33 mg/minute (Adults and Pediatric Patients 11 years and older) [see Dosage and Administration (2.2)]).
Acute and chronic oxalate nephropathy have occurred with prolonged administration of high doses of ascorbic acid [see Warnings and Precautions (5.1)]. In patients with glucose-6-phosphate dehydrogenase deficiency, severe hemolysis has occurred [seeWarnings and Precautions (5.2)].
-
7 DRUG INTERACTIONS
7.1 Antibiotics
Ascorbic acid may decrease activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin. Bleomycin is inactivated in vitro by ascorbic acid. If the antibiotic efficacy is suspected to be decreased by concomitant administration of ascorbic acid injection, discontinue ascorbic acid injection administration.
7.2 Amphetamines & Other Drugs Affected by Urine Acidification
Ascorbic acid may acidify the urine and lower serum concentrations of amphetamine by increasing renal excretion (as reflected by changes in amphetamine urine recovery rates). In case of decreased amphetamine efficacy, discontinue ascorbic acid injection administration. Standard monitoring of therapy is warranted.
In addition, acidification of urine by ascorbic acid will alter the excretion of certain drugs affected by the pH of the urine (e.g., fluphenazine) when administered concurrently. It has been reported that concurrent administration of ascorbic acid and fluphenazine has resulted in decreased fluphenazine plasma concentrations. Standard monitoring of therapy is warranted.
7.3 Warfarin
Limited case reports have suggested interference of ascorbic acid with the anticoagulation effects of warfarin; however, for patients on warfarin therapy treated with ascorbic acid doses up to 1000 mg/day (5 times the largest recommended single dose) for 2 weeks (twice the maximum recommended duration), no effect was observed. Standard monitoring for anti-coagulation therapy should continue during ascorbic acid treatment, as per standard of care.
7.4 Laboratory Test Interference
Because ascorbic acid is a strong reducing agent, it can interfere with numerous laboratory tests based on oxidation-reduction reactions (e.g., glucose, nitrite and bilirubin levels, leukocyte count, etc.). Chemical detecting methods based on colorimetric reactions are generally those tests affected. Ascorbic acid may lead to inaccurate results (false negatives) obtained for checking blood or urinary glucose levels, nitrite, bilirubin, and leukocytes if tested during or within 24 hours after infusion [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on use of ascorbic acid injection in pregnant women to inform a drug-associated risk of adverse developmental outcomes; however, use of ascorbic acid (vitamin C) has been used during pregnancy for several decades and no adverse developmental outcomes are reported in the published literature [see Data]. There are dose adjustments for ascorbic acid (vitamin C) use during pregnancy [see ClinicalConsiderations].
Animal reproduction studies have not been conducted with ascorbic acid injection.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
- Dose Adjustments During Pregnancy and Post-Partum Period
- Follow the U.S. Recommended Dietary Allowances (RDA) for pregnant women when considering use of Ascorbic acid injection for treatment of scurvy [see Dosage and Administration (2.3)].
Data
- Human Data
- There are no available data on use of ascorbic acid injection or another ascorbic acid injection in pregnant women. However, a published meta–analysis of randomized studies evaluating a large number of pregnant women who took oral ascorbic acid (vitamin C) (through diet and supplementation) at doses ranging from 500 to 1000 mg/day (2.5 to 5 times the recommended daily intravenous dose, respectively) [see Dosage andAdministration (2.3)] between the 9th and 16th weeks of pregnancy showed no increased risk of adverse pregnancy outcomes such as miscarriage, preterm premature rupture of membranes, preterm delivery or pregnancy induced hypertension when compared to placebo. These data cannot definitively establish or exclude the absence of a risk with ascorbic acid (vitamin C) during pregnancy.
8.2 Lactation
Risk Summary
There are no data on the presence of ascorbic acid (vitamin C) in human milk following intravenous dosing in lactating women. Ascorbic acid (vitamin C) is present in human milk after maternal oral intake. Maternal oral intake of ascorbic acid (vitamin C) exceeding the U.S. Recommended Dietary Allowances (RDA) for lactation does not influence the ascorbic acid (vitamin C) content in breast milk or the estimated daily amount received by breastfed infants. There are no data on the effect of ascorbic acid (vitamin C) on milk production or the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ascorbic acid injection and any potential adverse effects on the breastfed child from ascorbic acid injection or from the underlying maternal condition. Follow the U.S. Recommended Dietary Allowances (RDA) for lactating women when considering use of ascorbic acid injection for treatment of scurvy [see Dosage and Administration (2.3)].
8.4 Pediatric Use
Ascorbic acid injection is indicated for the short term (up to 1 week) treatment of scurvy in pediatric patients age 5 months and older for whom oral administration is not possible, insufficient or contraindicated. The safety profile of ascorbic acid in pediatric patients is similar to adults; however, pediatric patients less than 2 years of age may be at higher risk of oxalate nephropathy following ascorbic acid administration due to age-related decreased glomerular filtration [see Warnings and Precautions (5.1)].
Ascorbic acid injection is not indicated for use in pediatric patients less than 5 months of age.
8.5 Geriatric Use
Glomerular filtration rate is known to decrease with age and as such may increase risk for oxalate nephropathy following ascorbic acid administration in elderly population [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Ascorbic acid injection should be used with caution in scorbutic patients with a history of or risk of developing renal oxalate stones or evidence of renal impairment or other issues (e.g., patients on dialysis, patients with diabetic nephropathy, and renal transplant recipients). These patients may be at increased risk of developing acute or chronic oxalate nephropathy following high dose ascorbic acid administration [see Warning and Precautions(5.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Ascorbic acid injection, USP for intravenous use is a colorless to pale yellow, preservative-free, hypertonic, sterile, non-pyrogenic solution of ascorbic acid. Ascorbic acid injection must be diluted with an appropriate infusion solution (e.g., 5% Dextrose Injection, USP; Sterile Water for Injection, USP) [see Dosage and Administration (2.1)].
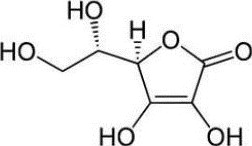
The chemical name of ascorbic acid, USP is L-ascorbic acid. The molecular formula is C6H8O6. It has the following structural formula:
Each ascorbic acid injection, USP, 50 mL, Pharmacy Bulk Package vial that contains 25,000 mg ascorbic acid, equivalent to 28,125 mg sodium ascorbate.
Each mL of ascorbic acid injection, USP contains 500 mg of ascorbic acid (equivalent to 562.5 mg of sodium ascorbate which amounts to 65 mg sodium/mL of ascorbic acid injection), 130 mg of sodium bicarbonate 0.28 mg of edetate disodium (dihydrate). Sodium hydroxide is added for pH adjustment (pH range 5.6 to 6.6). It contains no bacteriostatic or antimicrobial agent.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action of ascorbic acid for the treatment of symptoms and signs of scurvy (a disorder caused by severe deficiency in vitamin C) is unknown; however, administration of ascorbic acid in patients with scurvy is thought to restore the body pool of ascorbic acid.
12.3 Pharmacokinetics
In a single pharmacokinetic study, healthy male and female adults (n=8) were given a single intravenous dose of 1000 mg ascorbic acid (5 times the largest recommended single dose) infused over a 30 minute period. The mean peak exposure to ascorbic acid was 436.2 μM and occurred at the end of the 30 minute infusion.
Distribution
Ascorbic acid is distributed widely in the body, with large concentrations found in the liver, leukocytes, platelets, glandular tissues, and lens of the eye. Based on data from oral exposure, ascorbic acid is known to be distributed into breast milk and crosses the placental barrier.
Elimination
When the body is saturated with ascorbic acid, the plasma concentration will be about the same as that of the renal threshold; if further amounts are then administered, most of it is excreted in the urine. When body tissues are not saturated and plasma concentration is low, administration of ascorbic acid results in little or no renal excretion. The mean±SD (N=3) half-life observed in the single dose PK study, as described above, was 7.4±1.4 h.
- Metabolism
- A major route of metabolism of ascorbic acid involves its conversion to urinary oxalate, presumably through intermediate formation of its oxidized product, dehydroascorbic acid.
- Excretion
- There is a renal threshold for ascorbic acid (vitamin C); the vitamin is excreted by the kidney in large amounts only when the plasma concentration exceeds this threshold, which is approximately 1.4 mg/100 mL.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ascorbic acid injection, USP for intravenous use is a colorless to pale yellow solution supplied as:
Product
CodeUnit of Sale Strength
(Concentration)Each 207755 NDC: 65219-027-55
Unit of 125,000 mg per
50 mL
(500 mg per mL)NDC: 65219-027-55
50 mL Pharmacy Bulk
Package vial207750 NDC: 65219-027-50
Unit of 2525,000 mg per
50 mL
(500 mg per mL)NDC: 65219-027-05
50 mL Pharmacy Bulk
Package vialStore in a refrigerator at 2°C to 8°C (36°F to 46°F).
Protect from light. This product contains no preservative. See Dosage and Administration (2.1) for detailed instructions on preparation, dilution, and administration of ascorbic acid injection. Excursions to ambient conditions for up to 30 days during storage or shipping are acceptable.
The container closure is not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
- Inform patients that treatment with ascorbic acid injection may increase their risk of oxalate nephropathy [see Warnings and Precautions (5.1)].
- Inform patients that treatment with ascorbic acid injection may impact laboratory results, including blood and urine glucose tests, up to 24 hours after infusion [see Warnings and Precautions (5.3)].
- Inform patients with glucose-6-phosphate dehydrogenase deficiency that treatment with ascorbic acid injection may increase their risk of hemolysis [see Warnings and Precautions (5.2)].
Manufactured by:
Lake Zurich, IL 60047
www.fresenius-kabi.com/us
451857
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Ascorbic Acid Injection, USP 50 mL Pharmacy Bulk Package Vial Label
NDC: 65219-027-55 Sterile
Ascorbic Acid
Injection, USP
25,000 mg per 50 mL
(500 mg per mL)
Pharmacy Bulk Package –
Not for Direct Infusion
For Intravenous Use Only.
Any Unused Portion Must be
Discarded Within 4 Hours.
50 mL Rx only
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Ascorbic Acid Injection, USP 50 mL Pharmacy Bulk Package Carton
Sterile NDC: 65219-027-55 207755
Ascorbic Acid
Injection, USP
25,000 mg per 50 mL
(500 mg per mL)
Pharmacy Bulk Package –
Not for Direct Infusion
For Intravenous Use Only.
Any Unused Portion Must be
Discarded Within 4 Hours.
Contains No Preservative.
50 mL Rx only
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Ascorbic Acid Injection, USP 50 mL Pharmacy Bulk Package Vial Label
NDC: 65219-027-05 Sterile
Ascorbic Acid
Injection, USP
25,000 mg per 50 mL
(500 mg per mL)
Pharmacy Bulk Package –
Not for Direct Infusion
For Intravenous Use Only.
Any Unused Portion Must be
Discarded Within 4 Hours.
50 mL Rx only
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Ascorbic Acid Injection, USP 50 mL Pharmacy Bulk Package Tray Label
Sterile NDC: 65219-027-50 207750
Ascorbic Acid
Injection, USP
25,000 mg per 50 mL
(500 mg per mL)
Pharmacy Bulk Package –
Not for Direct Infusion
For Intravenous Use Only.
25 x 50 mL
Pharmacy Bulk Package Vials Rx only
-
INGREDIENTS AND APPEARANCE
ASCORBIC ACID
ascorbic acid injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-027 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) 130 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.28 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-027-55 1 in 1 CARTON 12/08/2025 1 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 65219-027-50 25 in 1 TRAY 12/08/2025 2 NDC: 65219-027-05 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217131 12/08/2025 Labeler - Fresenius Kabi USA, LLC (013547657) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 ANALYSIS(65219-027) , MANUFACTURE(65219-027)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.