Menthol Pain Relief Gel Patch
Menthol Pain Relief Gel Patch by
Drug Labeling and Warnings
Menthol Pain Relief Gel Patch by is a Otc medication manufactured, distributed, or labeled by Twin Med LLC, DR. SABHARWAL'S WOUND CARE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
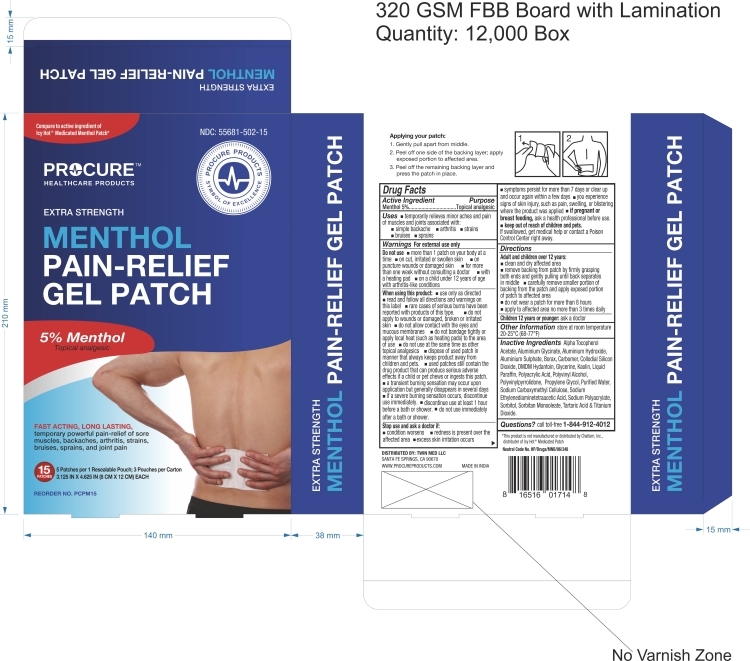
MENTHOL PAIN RELIEF GEL PATCH- menthol pain relief gel patch patch
Twin Med LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Menthol Pain Relief Gel Patch
Use
Temporarily relieves minor aches and pain of muscles and joints accociated with, simple backache, arthritis, strains, bruises and sprains
Do not use:
- more than 1 patch on your body at a time
- on cut, irritated or swollen skin
- on puncture wounds or damaged skin
- for more than one week without consulting a doctor
- with heating pad
- on a child under 12 yeas of age with arthritis-like conditions
When using this product:
- use only as directed.
- read and follow all directions and warnings on this label
- rare cases of serious burns have been reported with products of this type.
- do not apply to wounds or damaged, broken or irritated skin
- do not allow contact with eyes and mucous membranes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not use at the same time as other topical analgesics
- dispose of used patch in manner that always keeps product away from children and pets
- used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch
- a transient burning sensation may occur upon application but generally disappears in several days
- if a severe burning sensation occurs, discontinue use immediately.
- discontinue use at least 1 hour before a bath or shower.
- do not use immediately after a bath or shower.
Stop use and ask a doctor if:
condition worsens
redness is present over the affected area
excess skin irritation occurs
symptoms persist for more than 7 days or clear up and occur again within a few days
you experience signs of skin injury such as pain, swelling, or blistering where the product was applied
keep out of reach of children and pets
if swallowed, get medical help or contact a Poison Control Center right away
Directions:
Adult and children over 12years:
- Clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until back separates in middle
- carefully remove smaller portion of backing from the patch and apply exposed portion of patch to affected area
- do not wear a patch for more than 8 hours
- apply affected area no more than 3 times daily
- children 12 years or younger: ask a doctor
Other Information:
Store at room temperature 20-250C (68-770F)
Questions? call toll-free 1-844-912-4012
Inactive Ingredients:
Alpha Tocopherol, Aluminum Sulphate, Borax, Carbomer, Colloidal Silicon Dioxide, DMDM Hydantoin, Glycerin, Kaolin, Polyacrylic Acid, Polyvinyl Alcohol, Polyvinylpyrrolidone , Propylene Glycol, Purified Water, Sodium Carboxymethyl Cellulose, Sodium Ethylenediaminetetraacetic Acid, Sodium Polyacrylate, Sorbitol, Sorbitol Monooleate , Tartaric Acid & Titanium Dioxide, Paraffin
| MENTHOL PAIN RELIEF GEL PATCH
menthol pain relief gel patch patch |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Twin Med LLC (009579330) |
| Registrant - Twin Med LLC (009579330) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DR. SABHARWAL'S WOUND CARE | 862184668 | manufacture(55681-502) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.