TYLENOL REGULAR STRENGTH- acetaminophen tablet
TYLENOL by
Drug Labeling and Warnings
TYLENOL by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. The maximum daily dose of this product is 10 tablets (3,250 mg) in 24 hours for adults or 5 tablets (1,625 mg) in 24 hours for children.
Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 tablets every 4 to 6 hours while symptoms last
- do not take more than 10 tablets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
children 6 years to under 12 years - take 1 tablet every 4 to 6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- do not use for more than 5 days unless directed by a doctor
children under 6 years ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 50580-496-60
TYLENOL®
Acetaminophen
Pain Reliever
Fever ReducerRegular Strength
Actual Size
100 Tablets
325 mg each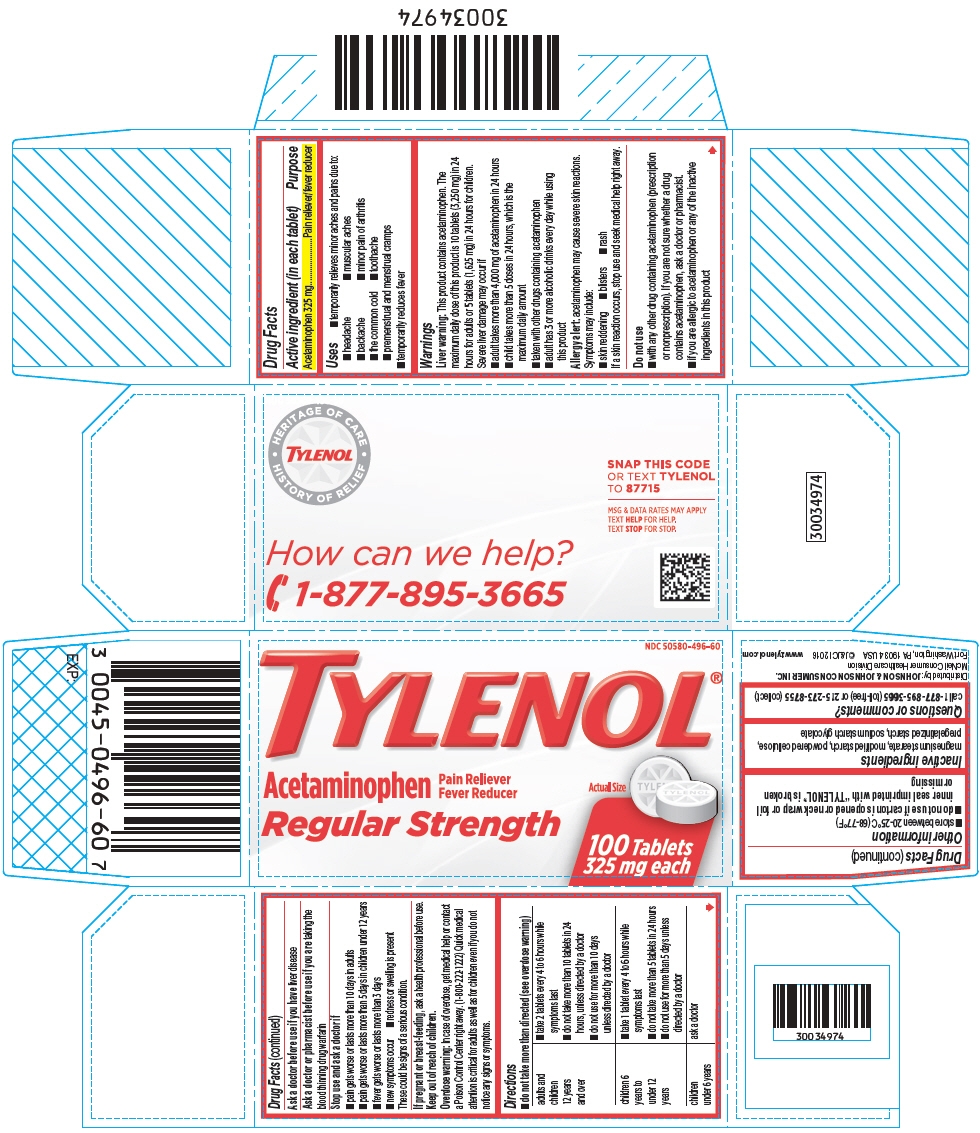
-
INGREDIENTS AND APPEARANCE
TYLENOL REGULAR STRENGTH
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50580-496 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 325 mg Inactive Ingredients Ingredient Name Strength magnesium stearate (UNII: 70097M6I30) powdered cellulose (UNII: SMD1X3XO9M) sodium starch glycolate type a potato (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code TYLENOL;325 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50580-496-60 1 in 1 CARTON 02/01/1999 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 50580-496-98 1 in 1 CARTON 05/31/2019 2 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 02/01/1999 Labeler - Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division (878046358)
Trademark Results [TYLENOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TYLENOL 98777610 not registered Live/Pending |
Kenvue Inc. 2024-09-30 |
 TYLENOL 97348733 not registered Live/Pending |
Johnson & Johnson 2022-04-06 |
 TYLENOL 90512746 not registered Live/Pending |
Johnson & Johnson 2021-02-05 |
 TYLENOL 88182835 not registered Dead/Abandoned |
Johnson & Johnson 2018-11-06 |
 TYLENOL 85962420 4874809 Live/Registered |
Johnson & Johnson 2013-06-18 |
 TYLENOL 77564801 4096488 Live/Registered |
JOHNSON & JOHNSON 2008-09-08 |
 TYLENOL 77563361 not registered Dead/Abandoned |
Johnson & Johnson 2008-09-05 |
 TYLENOL 76072484 2660253 Live/Registered |
JOHNSON & JOHNSON 2000-06-16 |
 TYLENOL 73824422 1621973 Live/Registered |
JOHNSON & JOHNSON 1989-09-11 |
 TYLENOL 72326412 0890360 Live/Registered |
MCNEIL LABORATORIES, INCORPORATED 1969-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.