Instant Hand Antiseptic
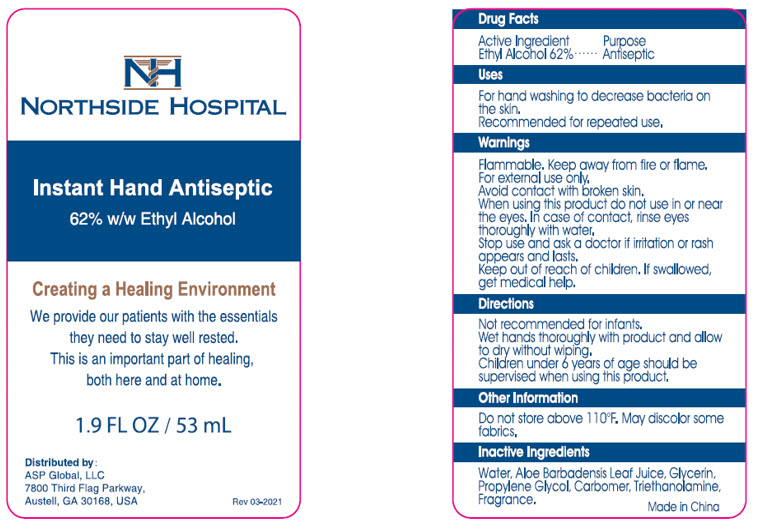
Drug Facts
Active Ingredient
Ethyl Alcohol 62%
Uses
For hand washing to decrease bacteria on the skin.
Recommended for repeated use.
Warnings
Flammable. Keep away from fire or flame.
For external use only.
Avoid contact with broken skin.
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help.
Directions
Not recommended for infants.
Wet hands thoroughly with product and allow to dry without wiping.
Children under 6 years of age should be supervised when using this product.
Other Information
Do not store above 110°F. May discolor some fabrics.
Inactive Ingredients
Water, Aloe Barbadensis Leaf Juice, Glycerin, Propylene Glycol, Carbomer, Triethanolamine, Fragrance.
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway,
Austell, GA 30168, USA
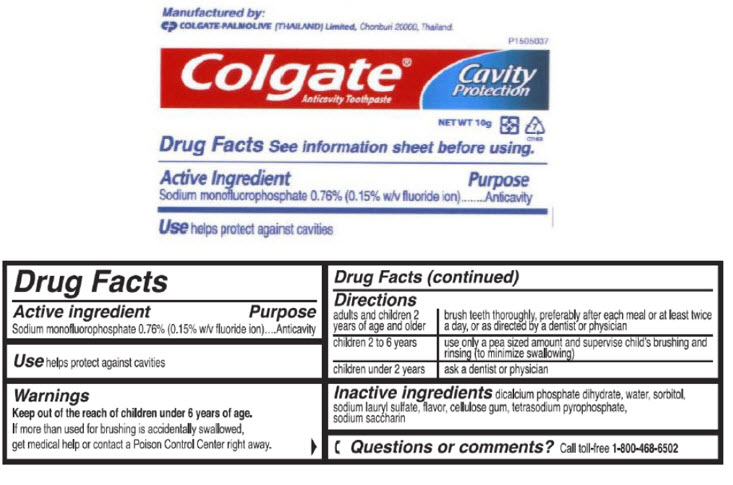
Colgate® Anticavity Toothpaste
Drug Facts
Active ingredient
Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion)
Use
helps protect against cavities
Warnings
Keep out of the reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
dicalcium phosphate dihydrate, water, sorbitol, sodium lauryl sulfate, flavor, cellulose gum, tetrasodium pyrophosphate, sodium saccharin
Questions or comments?
Call toll-free 1-800-468-6502
Manufactured by:
COLGATE-PALMOLIVE (THAILAND) Limited, Chonburi 20000, Thailand.
PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
NORTHSIDE HOSPITAL
Instant Hand Antiseptic
62% w/w Ethyl Alcohol
Creating a Healing Environment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.
1.9 FL OZ / 53 mL
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway,
Austell, GA 30168, USA
Rev 03-2021
PRINCIPAL DISPLAY PANEL - 10 g Tube Label
P1505037
Colgate®
Anticavity Toothpaste
Cavity
Protection
NET WT 10g
7
OTHER
PRINCIPAL DISPLAY PANEL - Kit Label
Components Made in China:
Shampoo/Conditioner, Body Wash, Body Lotion,
Alcohol-Free Mouthwash, Lip Balm, Hand Sanitizer,
Hairbrush, Toothbrush, Ear Plugs, Eye Mask, Face
Mask, Facial Tissue, Pen, Puzzle Book, Questions
for My Care Team Book, Insulated Mug, Cup, Cleansing
Bottle, Disposable Panties/Briefs, Emesis Bag, Sanitary
Pad
Component Made in Thailand:
Toothpaste
Bag Made in China and Assembled in the USA:
H.E.A.R.T Strings Bag
Lot #:
Exp:
1000 Johnson Ferry Rd NE.
Atlanta, GA 30342
Tel: (404) 851-8000
NORTHSIDE HOSPITAL
H.E.A.R.T. Strings Bag
NSMTKTB01-A
Creating a Healing Environment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.
Contents/Origins(On Back)
- Northside Amenity Kit - Main
includes: 2.0 fl.oz. Shampoo/Conditioner,
2.0 fl.oz. Body Wash, 2.0 fl.oz. Body Lotion,
2.0 fl.oz. Alcohol-Free Mouthwash, 1.9 fl.oz.
Hand Sanitizer, 0.15 oz. net wt. Lip Balm,
Hairbrush, Toothbrush, Toothpaste, Ear Plugs,
Eye Mask, Face Mask, Facial Tissue, Pen, Puzzle
Book, Questions for My Care Team Book
- Insulated Mug
includes: 1 Insulated Mug with Lid and Straw,
30 oz.
- Disposable Panties/Briefs
includes: 6 Disposable Panties/Briefs
- Sanitary Pad
includes: 6 Sanitary Pads, 6.5" x 13.5"
- Cleansing Bottle
includes: 1 Cleansing Bottle, 8 oz.
- Cup
includes: 1 Cup, 8 oz.
- Emesis Bag
includes: 1 Emesis Bag, Easy Lock
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway,
Austell, GA 30168, USA
Rev 00
NORTHSIDE HOSPITAL
Item #: NSMTKTB01-A
Description: KIT, NORTHSIDE HEART STRINGS
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 6 KIT/CS
Carton #: XXX of XXX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: 23 x 18 x 16 INCH
Components Made in China:
Shampoo/Conditioner, Body Wash, Body Lotion,
Alcohol-Free Mouthwash, Lip Balm, Hand Sanitizer,
Hairbrush, Toothbrush, Ear Plugs, Eye Mask, Facial
Tissue, Face Mask, Pen, Puzzle Book, Questions for
My Care Team Book, Insulated Mug, Cup, Cleansing
Bottle, Emesis Bag, Disposable Panties/Briefs,
Sanitary Pad
Component Made in Thailand:
Toothpaste
Bag Made in China and Assembled in the USA:
H.E.A.R.T. Strings Bag
NORTHSIDE HOSPITAL
NORTHSIDE HOSPITAL