PANOXYL- benzoyl peroxide cream
PanOxyl by
Drug Labeling and Warnings
PanOxyl by is a Otc medication manufactured, distributed, or labeled by Crown Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- wet area to be cleansed
- apply acne wash and gently massage area for 1-2 minutes
- rinse thoroughly and pat dry
- because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other information
-
Inactive ingredients
carbomer homopolymer type C, carbomer interpolymer type A, decyl glucoside, dimethicone, dioctyl sodium sulfosuccinate, glycerin, palmitic acid, polyacrylate crosspolymer-6, polyoxyl 40 stearate, propanediol, purified water, silica, sodium chloride, sodium citrate, sodium hydroxide, sodium laurylglucosides hydroxypropylsulfonate, sorbitan stearate, sorbitol, stearic acid, t-butyl alcohol, xanthan gum
- Questions or comments?
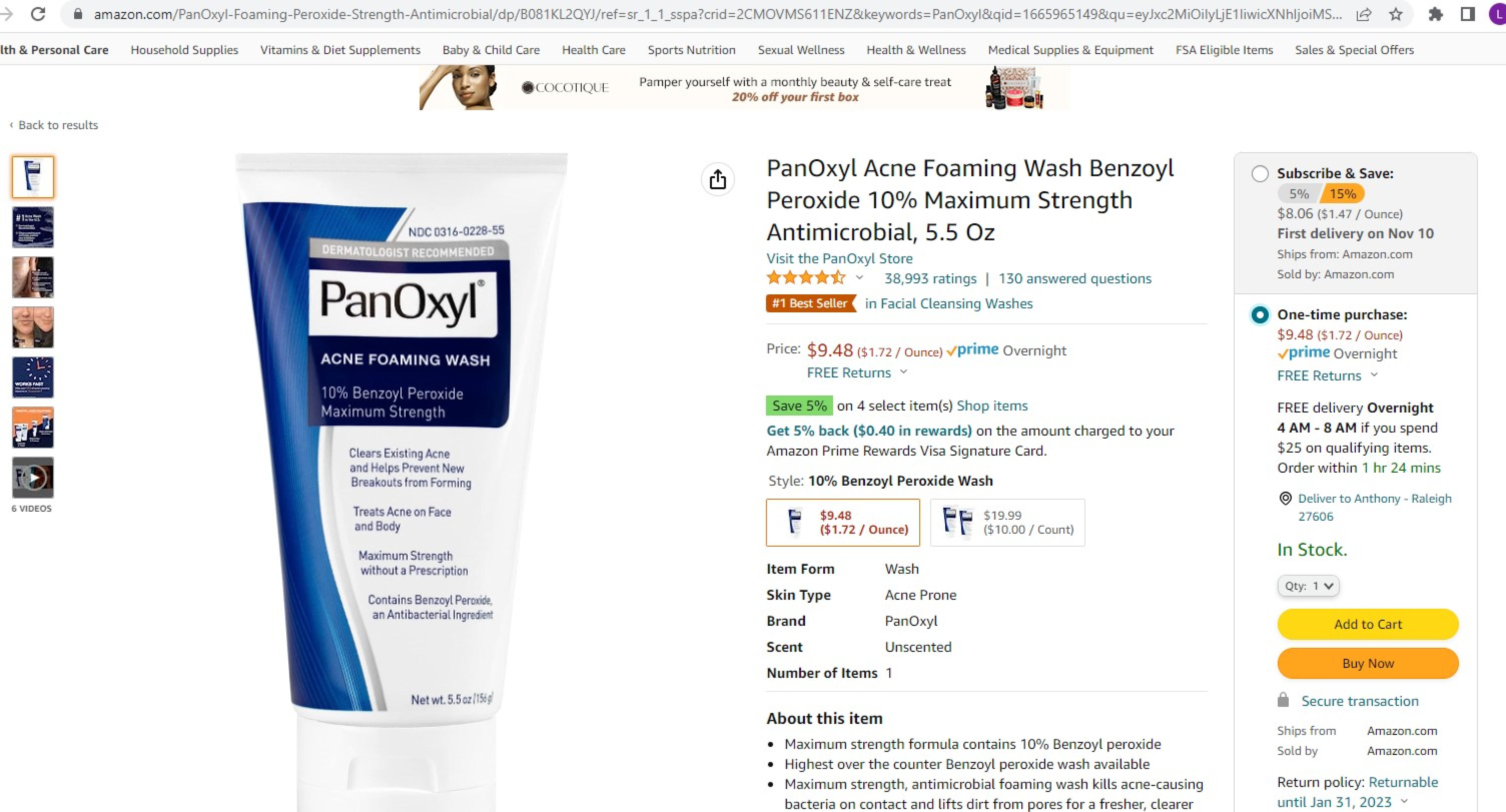
- Panoxyl 10% Tube
- Panoxyl 10% Carton
-
INGREDIENTS AND APPEARANCE
PANOXYL
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0316-0228 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIMETHICONE (UNII: 92RU3N3Y1O) DOCUSATE SODIUM (UNII: F05Q2T2JA0) PALMITIC ACID (UNII: 2V16EO95H1) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) STEARIC ACID (UNII: 4ELV7Z65AP) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) PROPANEDIOL (UNII: 5965N8W85T) SODIUM LAURYLGLUCOSIDES HYDROXYPROPYLSULFONATE (UNII: Z6GFR7R72Y) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0316-0228-01 1 in 1 CARTON 10/12/2022 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0316-0228-03 85 g in 1 TUBE; Type 0: Not a Combination Product 11/12/2022 3 NDC: 0316-0228-55 1 in 1 CARTON 12/01/2018 3 156 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/25/2011 Labeler - Crown Laboratories (119508400) Establishment Name Address ID/FEI Business Operations Crown Laboratories 119508400 manufacture(0316-0228)
Trademark Results [PanOxyl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PANOXYL 98758809 not registered Live/Pending |
Crown Laboratories, Inc. 2024-09-19 |
 PANOXYL 98730203 not registered Live/Pending |
Foshan Taodu E-commerce Co., Ltd 2024-09-03 |
 PANOXYL 72437359 0966901 Live/Registered |
STIEFEL LABORATORIES, INC. 1972-10-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

