GENTLECARE HAND AND SKIN SANITIZER- benzalkonium chloride liquid
GENTLECARE HAND AND SKIN SANITIZER by
Drug Labeling and Warnings
GENTLECARE HAND AND SKIN SANITIZER by is a Otc medication manufactured, distributed, or labeled by PARAGON SOLUTIONS US, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
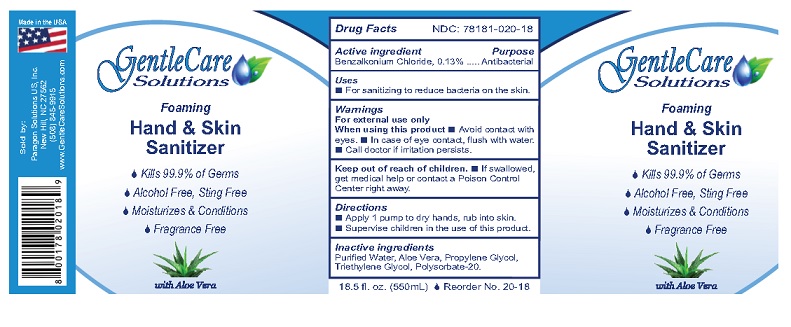
Principal Display Panel
GentleCare
Solutions
Foaming
Hand & Skin
Sanitizer- Kills 99.99% of Germs
- Moisturizes & Conditions
- Alcohol Free, Sting Free
- Fragrance Free
with Aloe Vera
1.7 fl. oz. (50 mL)Sold by:
GentleCare
SolutionsA division of:
Paragon Solutions US, Inc.
New Hill, NC 27562www.gentlecare.us
Questions?
Call Us Toll Free
1.800.262.8670
Monday to Friday
9:00 am to 4:00 pm EST1.7 FL OZ

7.5 FL OZ

16.5 FL OZ
1 GAL

res
-
INGREDIENTS AND APPEARANCE
GENTLECARE HAND AND SKIN SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 78181-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA FLOWER (UNII: 575DY8C1ER) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRIETHYLENE GLYCOL (UNII: 3P5SU53360) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 78181-020-02 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/01/2020 2 NDC: 78181-020-07 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/01/2020 3 NDC: 78181-020-18 550 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/01/2020 4 NDC: 78181-020-28 3785 mL in 1 JUG; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/01/2020 Labeler - PARAGON SOLUTIONS US, INC. (069788314) Registrant - PARAGON SOLUTIONS US, INC. (069788314) Establishment Name Address ID/FEI Business Operations Lifequest Creations LLC 053654857 manufacture(78181-020)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.