CHLORAPREP SWABSTICK- chlorhexidine gluconate and isopropyl alcohol solution
ChloraPrep Swabstick by
Drug Labeling and Warnings
ChloraPrep Swabstick by is a Otc medication manufactured, distributed, or labeled by CareFusion 213 LLC, Becton, Dickinson and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- BD ChloraPrep™ Single Swabstick 1.75 mL Applicators Active ingredients
- Purpose
- Use
- Warnings
- Allergy alert:
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- maximal treatment area for one applicator
- one single swabstick applicator is approximately 2.5 in. x 2.5 in. (42 cm 2)
- tear pouch at side notch to reveal applicator handles. Do not touch foam applicator tip. Place foam flat side down on the treatment area.
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): use repeated back-and-forth strokes for 30 seconds. (When using the triple swabstick applicators, use each swabstick sequentially within the 30 seconds). Allow the area to air dry for approximately 30 seconds. Do not blot or wipe away.
- moist surgical sites (e.g., inguinal fold): use repeated back-and-forth strokes for 2 minutes. (When using the triple swabstick applicators, use each swabstick sequentially within the 2 minutes). Allow the area to air dry for approximately 1 minute. Do not blot or wipe away.
- discard the applicator after a single use along with any portion of the solution not required to cover the prep area. It is not necessary to use the entire amount available.
- Other information
- Inactive ingredient
- Questions?
- BD ChloraPrep™ Triple Swabstick 5.25 mL Applicators Active ingredients
- Purposes
- Use
- Warnings
- Allergy alert
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- maximal treatment area using three swabsticks sequentially is approximately 5 in. x 5 in. (160 cm 2)
- tear pouch at side notch to reveal applicator handles. Do not touch foam applicator tip. Place foam flat side down on the treatment area.
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): using each swabstick sequentially, use repeated back-and-forth strokes for a total of 30 seconds. Allow the area to air dry for approximately 30 seconds. Do not blot or wipe away.
- moist surgical sites (e.g., inguinal fold): using each swabstick sequentially, use repeated back-and-forth strokes for a total of 2 minutes. Allow the area to air dry for approximately 1 minute. Do not blot or wipe away.
- discard the applicator after a single use along with any portion of the solution not required to cover the prep area. It is not necessary to use the entire amount available.
- Other information
- Inactive ingredient
- Questions?
-
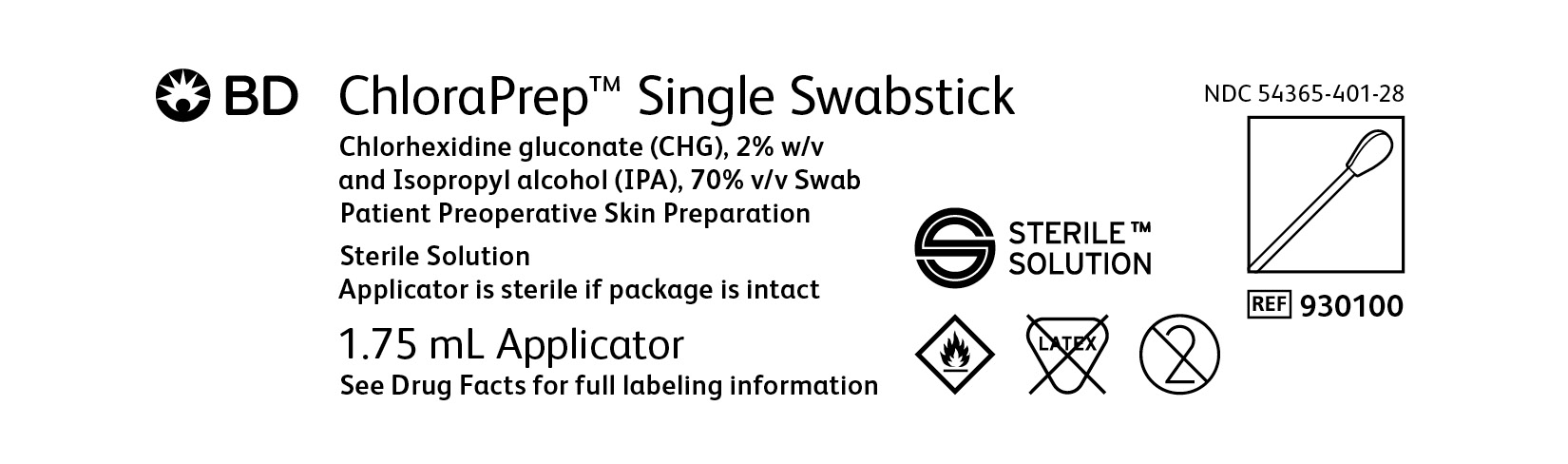
BD ChloraPrep™ Single Swabstick
Package/Label Principal Display Panel

PRINCIPAL DISPLAY PANEL-CARTON
BD ChloraPrep™ Single Swabstick
Chlorhexidine gluconate (CHG), 2% w/v
and Isopropyl alcohol (IPA), 70% v/v Swab
Patient Preoperative Skin Preparation
Sterile Solution
Applicator is sterile if package is intact
1.75 mL Applicators
NDC: 054365-401-28
Ref 930100
-
BD ChloraPrep™ Triple Swabsticks
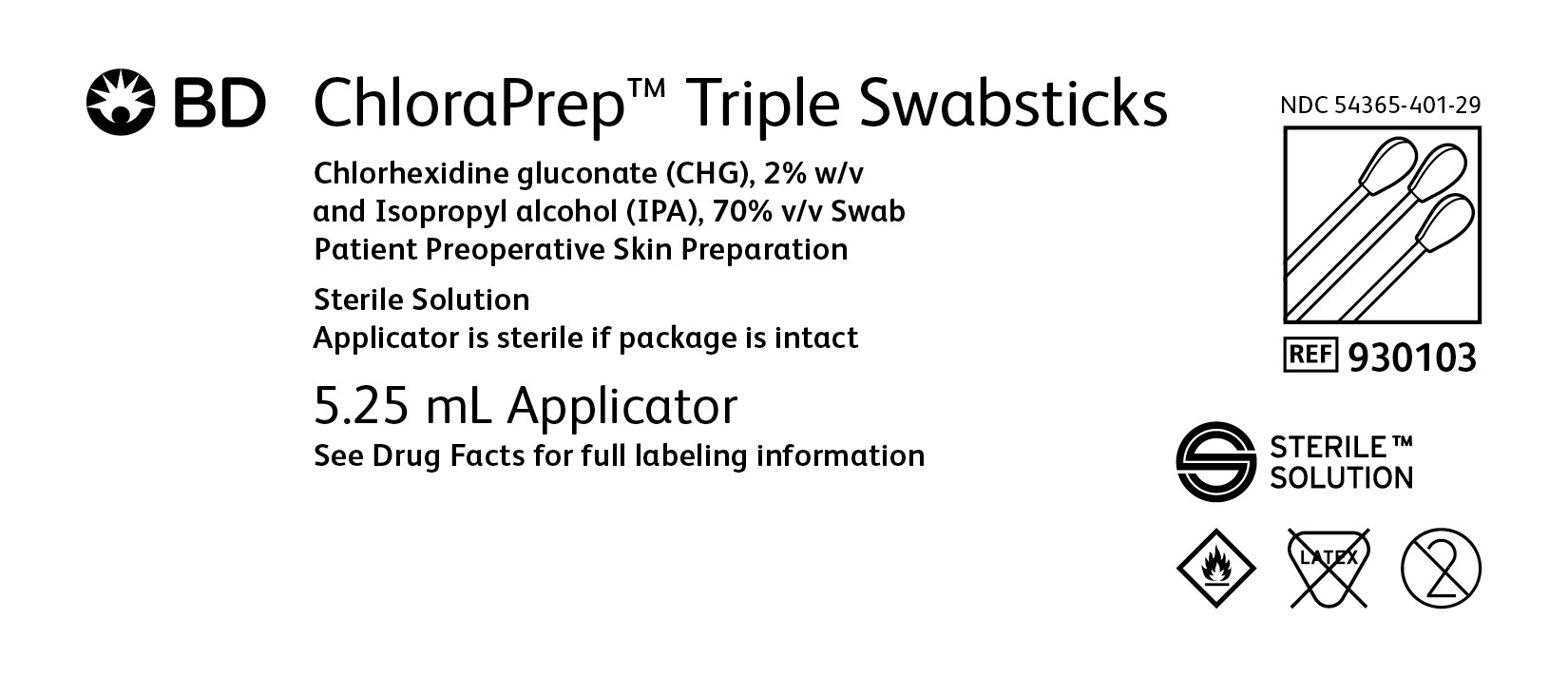

Chlorhexidine gluconate (CHG), 2% w/v and Isopropyl alcohol (IPA), 70% v/v Swab
Patient Preoperative Skin Preparation
Sterile Solution
Applicator is sterile if package is intact
5.25 mL Applicators
NDC: 54365-401-29
REF 930103

-
INGREDIENTS AND APPEARANCE
CHLORAPREP SWABSTICK
chlorhexidine gluconate and isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54365-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54365-401-28 48 in 1 CARTON 05/13/2024 1 1 in 1 POUCH 1 1.75 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC: 54365-401-29 40 in 1 CARTON 05/13/2024 2 3 in 1 POUCH 2 5.25 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021555 05/13/2024 Labeler - CareFusion 213 LLC (826496312) Registrant - Becton, Dickinson and Company (832696038) Establishment Name Address ID/FEI Business Operations CareFusion 213 LLC 826496312 manufacture(54365-401) , analysis(54365-401) , label(54365-401) , pack(54365-401) , sterilize(54365-401)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.