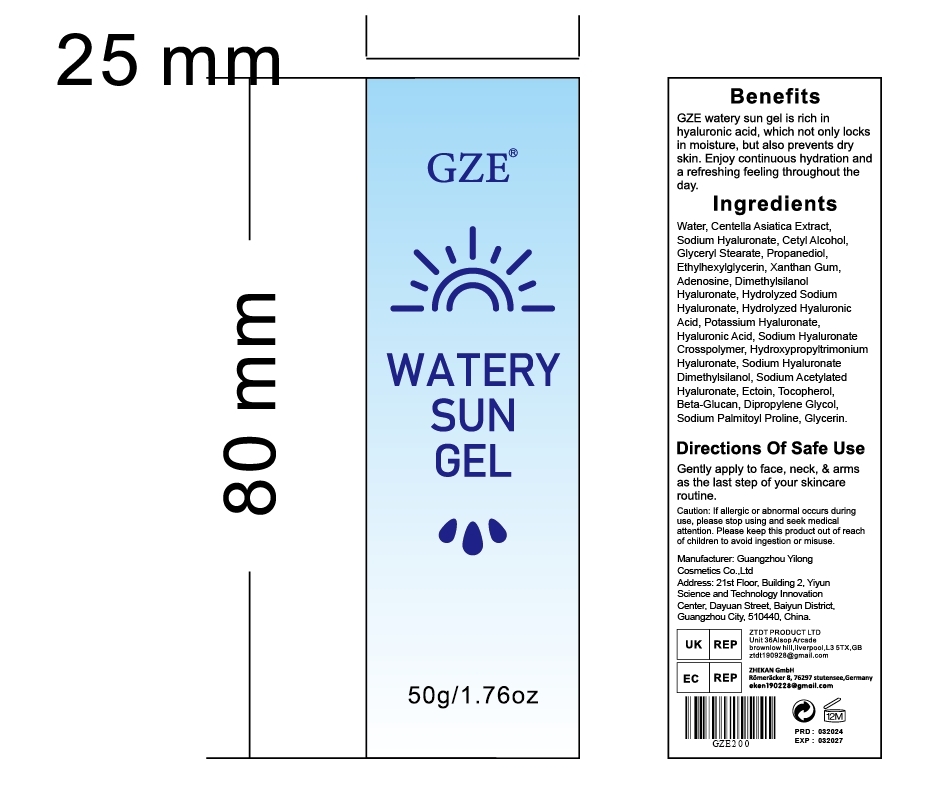
Gze Watery Sun Gel by Guangzhou Yilong Cosmetics Co.,Ltd.
Gze Watery Sun Gel by
Drug Labeling and Warnings
Gze Watery Sun Gel by is a Otc medication manufactured, distributed, or labeled by Guangzhou Yilong Cosmetics Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GZE WATERY SUN GEL- centella asiatica extract, hyaluronic acid gel
Guangzhou Yilong Cosmetics Co.,Ltd.
----------
Active ingredients.
Centella Asiatica Extract 15.17%
Sodium Hyaluronate 10%
Tocopherol 1%
Glycerin 1%
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
GLYCERIN
SODIUM ACETYLATED HYALURONATE
HYALURONIC ACID
WATER
PEG-9 DIGLYCIDYL ETHERISODIUM HYALURONATE CROSSPOLYMER
TOCOPHEROL
| GZE WATERY SUN GEL
centella asiatica extract, hyaluronic acid gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Guangzhou Yilong Cosmetics Co.,Ltd. (712647107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Yilong Cosmetics Co.,Ltd. | 712647107 | manufacture(83566-121) | |
Revised: 11/2024
Document Id: 269af55a-57ee-27c8-e063-6394a90af104
Set id: 166b5b62-8fcb-1d35-e063-6394a90a7130
Version: 6
Effective Time: 20241110