ALBUMINEX- albumin human solution
ALBUMINEX by
Drug Labeling and Warnings
ALBUMINEX by is a Prescription medication manufactured, distributed, or labeled by BPL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALBUMINEX® 25% Albumin (human) - kjda safely and effectively. See full prescribing information for ALBUMINEX® 25%.
ALBUMINEX® 25% (human albumin) solution for injection. Initial U.S. Approval: [2018]INDICATIONS AND USAGE
ALBUMINEX 25% is a 25% albumin solution indicated for adults and children:
- Hypovolemia (1.1)
- Ascites (1.2)
- Hypoalbuminemia including from burns (1.3)
- Acute Nephrosis (1.4)
- Acute Respiratory Distress Syndrome (ARDS) (1.5)
- Cardiopulmonary Bypass (1.6)
Indication Dose Hypovolemia Adults: Initial dose of 25 g (including renal dialysis).
For acute liver failure: initial dose of 12 to 25 g. (2.1)Prevention of central volume depletion after paracentesis due to cirrhotic ascites Adults: 8 g for every 1000 mL of ascitic fluid removed. (2.1) Hypoalbuminemia including from burns Adults: 50 to 75 g
For pre- and post-operative hypoproteinemia: 50 to 75 g.
For burn therapy after the first 24 h: initial dose of 25 g and dose adjustment to maintain plasma protein concentration of 2.5 g per 100 mL.
Third space protein loss due to infection: initial dose of 50 to 100 g. (2.1)Acute nephrosis Adults: 25 g together with diuretic once a day for 7-10 days. (2.1) Adult respiratory distress syndrome (ARDS) Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days, if necessary. (2.1) Cardiopulmonary bypass procedures Adults: Initial dose of 25 g. (2.1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
ALBUMINEX 25% is a solution for infusion: (3)
- ALBUMINEX 25% contains 25 g per dL of human albumin 50 mL (12.5 g) and 100 mL (25 g) glass vials
CONTRAINDICATIONS
- Hypersensitivity to human albumin or the excipients
- Severe anemia or cardiac failure with normal or increased intravascular volume
WARNINGS AND PRECAUTIONS
- Suspicion of allergic or anaphylactic reactions requires immediate discontinuation of the injection and implementation of appropriate medical treatment. (5.1)
- Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient's volume status. Use with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient. (5.2)
- When concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. (5.3)
- Assessment of electrolytes, coagulation and hematology parameters, and hemodynamic status when albumin is administered. (5.4)
- Do not dilute with sterile water for injection. (5.5)
- This product is made from human plasma and may contain infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease agent. (5.6)
ADVERSE REACTIONS
The most common adverse reactions are rigors, hypotension/decreased BP, tachycardia/increased heart rate, pyrexia, feeling cold (chills), nausea, vomiting, dyspnea/bronchospasm, rash/pruritus. Stop the infusion if anaphylaxis, with or without shock is observed. (6)
To report SUSPECTED ADVERSE REACTIONS, contact BPL Inc. at 1-844-427-5872 or MedInfo@BPL-US.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hypovolemia
1.2 Ascites
1.3 Hypoalbuminemia including from burns
1.4 Acute Nephrosis
1.5 Acute Respiratory Distress Syndrome (ARDS)
1.6 Cardiopulmonary Bypass
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Hypervolemia
5.3 Laboratory Parameters
5.4 Clinical Hemodynamics Parameters
5.5 Pre-infusion Preparation
5.6 Infectious Diseases
6 ADVERSE REACTIONS
6.1 General
6.2 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Hypovolemia
ALBUMINEX 25% is indicated for restoration and maintenance of circulating blood volume where volume deficiency has been demonstrated, and use of a colloid is appropriate e.g. hypovolemia following shock due to trauma or sepsis, in surgical patients and in other similar conditions with volume deficiency when restoration and maintenance of circulating blood volume is required in both adult and pediatric patients. In pediatric patients to reverse hypovolemia and achieve normal capillary refill time. 1, 2, 3, 4, 5, 6, 7, 8
1.2 Ascites
ALBUMINEX 25% is indicated for prevention of central volume depletion and maintenance of cardiovascular function after large volume parencentesis in patients with liver cirrhosis or other chronic liver disease in adults and children. 9, 10, 11, 12
ALBUMINEX 25% infusion plus administration of vasoactive drugs is indicated in the treatment of type I hepatorenal syndrome. 6
For patients with spontaneous bacterial peritonitis, ALBUMINEX 25% is indicated as adjuvant treatment to antibiotic therapy.
1.3 Hypoalbuminemia including from burns
ALBUMINEX 25% is indicated in patients with severe burn injury (> 20% total body surface area), but not until at least 12 to 24 hours after the burn, in order to correct protein loss, decrease overall fluid requirements, decrease systemic edema and stabilize cardiovascular hemodynamics without fluid overload (initial resuscitation should be with crystalloids).8, 14
ALBUMINEX 25% is also indicated in patients with pre- or post-operative hypoproteinemia and for third space protein loss due to infection or burns.
1.4 Acute Nephrosis
ALBUMINEX 25% is indicated in patients with acute nephrosis in combination with loop diuretics to reinforce the diuretic therapeutic effect, which is reduced by hypoalbuminemia, and for the correction of reduced oncotic pressure. 15, 16
-
2 DOSAGE AND ADMINISTRATION
For intravenous administration only.
2.1 Dosage
The concentration of ALBUMINEX 25% used, its dosage, and infusion rate should be adjusted to the patient's individual requirements and clinical indication.
Indication Dose Hypovolemia Hypovolemia
Adults: Initial dose of 25 g.
If hemodynamic stability is not achieved within 15 to 30 minutes, an additional dose may be given.
For acute liver failure: initial dose of 12 to 25 g. An infusion rate of 1-2 mL per minute is usually indicated.
For renal dialysis; the initial dose should not exceed 25 g and patients should be carefully observed for signs of fluid overload.Prevention of central volume depletion after paracentesis due to cirrhotic ascites Adults: 8 g for every 1000 mL of ascitic fluid removed. Hypoalbuminemia including from burns Adults: 50 to 75 g
For pre- and post-operative hypoproteinemia: 50 to 75 g.
In burns, therapy usually starts with administration of large volumes of crystalloid solution to maintain plasma volume. After 24 hours: initial dose of 25 g and dose adjustment to maintain plasma protein concentration of 2.5 g per 100 mL or a serum protein concentration of 5.2 g per 100 mL.
Third space protein loss due to infection or burns: initial dose of 50 to 100 g. An infusion rate of 1-2 mL per minute is usually indicated in the absence of shock.
Treatment should always be guided by hemodynamic response.Acute nephrosis Adults: 25 g together with diuretic once a day for 7-10 days Adult respiratory distress syndrome (ARDS) Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days, if necessary. Cardiopulmonary bypass procedures Adults: Initial dose of 25 g. Additional amounts may be administered as clinically indicated. 2.3 Administration
- Visually inspect the solution for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not use if there are any particulates seen or if the solution is discolored.
- If a large volume is infused, ensure that the vial is at room temperature before infusion.
- Do not dilute with Sterile Water for Injection as hemolysis may occur. ALBUMINEX 25% may be diluted with 0.9% saline or 5% dextrose.
- Begin the infusion within 4 hours of piercing the vial stopper (the product does not contain any preservative).
- Adjust the rate of infusion according to the individual patient's hemodynamic and other physiological responses, using appropriate clinical monitoring.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Suspicion of allergic or anaphylactic reactions requires immediate discontinuation of the infusion and implementation of appropriate medical treatment.
5.2 Hypervolemia
Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient's volume status. At the first clinical signs of cardiovascular overload (headache, dyspnea, jugular venous distention, increased blood pressure), the infusion must be slowed or stopped immediately.
Use albumin with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient. Examples of such conditions are:
- Decompensated heart failure
- Hypertension
- Esophageal varices
- Pulmonary edema
- Hemorrhagic diathesis
- Severe anemia
- Renal and post-renal anuria
5.3 Laboratory Parameters
20% - 25% human albumin solutions are relatively low in electrolytes compared to 4% - 5% human albumin solutions so if comparatively large volumes are to be replaced, care must be taken to ensure adequate substitution of other blood constituents (coagulation factors, electrolytes, platelets and erythrocytes).
5.4 Clinical Hemodynamics Parameters
Colloid-osmotic effect of human albumin 25% is approximately five times that of blood plasma. Therefore, when concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. Patients should be monitored carefully to guard against circulatory overload and hyperhydration. Patients with marked dehydration require administration of additional fluids.
The following parameters should be assessed during administration of ALBUMINEX 25%:
- Arterial blood pressure and pulse rate
- Central venous pressure
- Pulmonary artery occlusion pressure
- Urine output
- Electrolytes
- Hematocrit/hemoglobin
5.5 Pre-infusion Preparation
ALBUMINEX 25% must not be diluted with sterile water for injection as this may cause hemolysis in recipients. The product can be diluted in an isotonic solution (e.g., 5% dextrose in water or 0.9% sodium chloride) [see Dosage and Administration (2.2)].
5.6 Infectious Diseases
Albumin is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for ALBUMINEX 25%.
-
6 ADVERSE REACTIONS
6.1 General
In general, human albumin solutions are well-tolerated and no specific, clinically relevant alterations in organ function or coagulopathy have been substantiated.26
The most common adverse reactions associated with infusion of human albumin solutions are rigors, hypotension/decreased BP, tachycardia/increased heart rate, pyrexia, feeling cold (chills), nausea, vomiting, dyspnea/bronchospasm, rash/pruritus.
Reactions usually resolve when the infusion is slowed or stopped.
Anaphylaxis, with or without shock, may occur and in this situation, stop the infusion.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with ALBUMINEX 25% use in pregnant women to inform on drug-associated risk. Animal reproduction studies have not been conducted using ALBUMINEX 25%. It is not known whether ALBUMINEX 25% can cause fetal harm when administered to a pregnant woman or can affect fertility. ALBUMINEX 25% should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ALBUMINEX 25% in human milk, the effects on the breast-fed infant, or the effects on milk production. The developmental and health benefits of breast-feeding should be considered along with the mother's clinical need for ALBUMINEX 25% and any potential adverse effects on the breast-fed infant from ALBUMINEX 25% or from the underlying maternal condition.
-
11 DESCRIPTION
ALBUMINEX 25% is a sterile, ready-for-use, clear, slightly viscous, almost colorless, yellow, amber or slightly green aqueous solution of human albumin for single dose intravenous infusion. It is prepared from the pooled plasma of US donors in FDA-licensed facilities in the US. The product also contains 130-160 mmol/L of sodium, less than 200 micrograms/L of aluminum and is stabilized with caprylate (0.08 mmol/g albumin) and acetyltryptophanate (0.08 mmol/g albumin) but does not contain any preservative.
12.5 g (50 mL) of ALBUMINEX 25% is oncotically equivalent to 250 mL plasma.
25 g (100 mL) of ALBUMINEX 25% is oncotically equivalent to 500 mL plasma.The vials are closed with a synthetic rubber stopper. The stopper is not made with natural rubber latex.
The viral risk from human plasma is minimized by the fractionation process and pasteurization of the albumin solution for 10 hours at 60°C (140°F) in its final container. These processes are effective for both enveloped and non-enveloped viruses. There have been no reports of virus transmission with products manufactured using this combination of processes. Typical reductions of experimental viral loads are shown in Table 1.
Table 1: Virus Reduction for Albumin (Human) 25% Mean Reduction Factors (log10) Enveloped Virus Enveloped Virus Enveloped Virus Enveloped Virus Non-Enveloped Virus Non-Enveloped Virus nd: not determined
HIV-1: Human Immunodeficiency Virus Type 1
BVDV: Bovine Viral Diarrhoea Virus
IBR: Infectious Bovine Rhinotracheitis
HAV: Hepatitis A Virus
CPV: Canine ParvovirusManufacturing Step HIV-1 Sindbis BVDV IBR HAV CPV A+1 Precipitation nd 4.1 >3.4 3.4 3.4 3.7 Fraction IV Precipitation >4.6 >7.1 >4.2 >5.7 4.2 6.0 Pasteurization >6.6 >6.2 >4.0 >5.0 4.7 4.2 Overall >11.2 >13.3 >8.2 >10.7 8.9 10.2 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Human albumin accounts for more than half of the total protein in the plasma and represents about 10% of protein synthesis activity by the liver. Human Albumin 25% has a corresponding hyperoncotic effect.
The primary physiological function of albumin results from its contribution to plasma colloid oncotic pressure and transport function. Albumin stabilizes circulating blood volume and is a carrier of hormones, enzymes, medicinal products and toxins.
Other physiological functions include antioxidant properties, free radical scavenging, and capillary membrane integrity.
12.3 Pharmacokinetics
Albumin is distributed throughout the extracellular space and more than 60% of the body albumin pool is located in the extravascular fluid compartment. Albumin has a circulating life span of 15-20 days, with a turnover of approximately 15 g per day. The balance between synthesis and breakdown is normally achieved by feedback regulation. Elimination is predominantly intracellular and due to lysosome proteases.
In healthy subjects, less than 10% of infused albumin leaves the intravascular compartment during the first 2 hours following infusion. There is considerable individual variation in the effect of albumin on plasma volume.
In some patients, the plasma volume can remain elevated for several hours. In critically ill patients, however, albumin can leak out of the vascular space in substantial amounts at an unpredictable rate.
-
15 REFERENCES
- Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;3650:2247-2256
- Bunn F, Trivedi D, Ashraf S. Colloid solutions for fluid resuscitation. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No.: CD001319. DOI: 10.1002/14651858.CD001319.pub3
- Powell-Tuck J, Gosling P, Lobo DN, et al. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients. BAPEN; 2011. Available from: http://www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf (Guideline Ref ID GIFTASUP2011)
- Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 2014;161(5):347-355
- Wiedermann CJ, Joannidis M. Albumin replacement in severe sepsis or septic shock. N Engl J Med 2014;371:83-84
- Akech S, Gwer S, Idro R, et al. Volume expansion with albumin compared to gelofusine in children with severe malaria: results of a controlled trial. PLoS Clin Trials 2006; 5:0001-0011
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine, 2013; 41(2):580–637
- Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systemic Reviews 2013, Issue 2.Art. No.CD000567. DOI:10.1002/14651858.CD000567.pub6.
- Runyon BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases 2012. http://www.aasld.org/publications/practice-guidelines-0 [accessed 15 April 2016]
- EASL clinical practise guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397–417
- Bernardi M, Carceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 2012;55:1172-1181
- Sarma MS, Yachha SK, Bhatia V, et al. Safety, complications and outcome of large volume paracentesis with or without albumin therapy in children with severe ascites due to liver disease. J Hepatol 2015:63;1126-1132
- Wiest R, Krag A, Gerbes A. Spontaneous Bacterial Peritonitis. Recent Guidelines and Beyond. Gut 2012; 61(2):297-310
- Pham TN, Cancio LC, Gibran NS. American Burn Association Practice Guidelines Burn Shock Resuscitation. J Burn Care Res 2008; 29(1): 257-266
- Bircan Z, Kervancioglu M. Does albumin and furosemide therapy affect plasma volume in nephrotic children? Pediatr Nephrol 2001;16:497-499
- Dharmaraj R, Hari P, Bagga A, Randomized cross-over trial comparing albumin and frusemide infusions in nephrotic syndrome. Pediatr Nephrol 2009; 24(4):775-782
- Martin GS, Moss M, Wheeler AP, et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med 2005;33(8):1681-1687
- Uhlig C, Pedro L, Silva PL, et al. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2014, 18:R10 http://ccforum.com/content/18/1/R10 [accessed 02 November 2016]
- Quinlan GJ, Mumby S, Martin GS, et al. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med 2004;32:755–759
- Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a metaanalysis of postoperative bleeding. Ann Thorac Surg 2001;72(2):527-534
- Golab HD, Scohy TV, de Jong PL, et al. Relevance of colloid oncotic pressure regulation during neonatal and infant cardiopulmonary bypass: a prospective randomized study. Eur J Cardio-Thor Surg 2011; 39(6):886-891
- Hanart C, Khalife M, De Villé A, et al. Perioperative volume replacement in children undergoing cardiac surgery: Albumin versus hydroxyethyl starch 130/0.4. Crit Care Med 2009;37 (2): 696-701 DOI: 10.1097/CCM.0b013e3181958c81
- Loeffelbein F, Zirell U, Benk C, et al. High colloid oncotic pressure priming of cardiopulmonary bypass in neonates and infants: implications on haemofiltration, weight gain and renal function. Eur J Cardio-Thor Surg 2008; 34(3):648-652
- Oliver WC Jr, Beynen FM, Nuttall GA, et al. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Ann Thor Surg 2003;75(5):1506-1512
- Yu K, Liu Y, Hei F, et al. Effect of different albumin concentrations in extracorporeal circuit prime on perioperative fluid status in young children. ASAIO Journal 2008; 54(5):463-466
- German Medical Association. Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives. 4th revised and updated edition 2014. http://www.bundesaerztekammer.de/aerzte/medizin-ethik/wissenschaftlicher-beirat/veroeffentlichungen/haemotherapie-transfusionsmedizin/english/ [accessed 15 April 2016]
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How ALBUMINEX 25% is supplied
ALBUMINEX 25%, 25 g/dL in clear Type II glass vials.
Strength Grams and fill size NDC carton number NDC vial number 25% 12.5 g in 50 mL 64208-2512-3 64208-2512-4 25% 25 g in 100 mL 64208-2512-7 64208-2512-8 Not all pack sizes may be marketed.
Storage and handling
Do not store above 30°C (86°F).
Keep the vial stored in the outer carton in order to protect from light.
Do not freeze.
Do not use ALBUMINEX 25% after the expiration date which is stated on the carton and label after "EXP." The expiration date refers to the last day of that month.
ALBUMINEX 25% should be inspected visually for particulate matter and discoloration prior to administration.
U.S. federal law prohibits dispensing without prescription.
-
17 PATIENT COUNSELING INFORMATION
Ensure that patients to be treated with ALBUMINEX 25% are informed of the potential risks and benefits of its use for their clinical condition [see Warnings and Precautions (5)].
Check that they are not known to be allergic to the product or its excipients [see Contraindications (4) and Description (11)].
Make them aware of the symptoms of anaphylaxis [see Hypersensitivity (5.1)].
Make them aware of the symptoms of potential circulatory overload [see Hypervolemia (5.2).
Inform patients that because ALBUMINEX 25% is derived from human blood plasma it may contain infectious agents that cause disease (e.g. viruses and, theoretically CJD agent) although the risk of infection from ALBUMINEX 25% has been reduced by the procedures used in donor selection and during manufacture [see Infectious Diseases (5.6) and Description (11)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 mL Vial Label
NDC: 64208-2512-8
Albumin (Human) - kjda
ALBUMINEX® 25%
solution for infusion
For intravenous use onlyDistributor: BPL USA, Inc., 302 E. Pettigrew Street,
Suite C-190, Durham, NC 27701 USA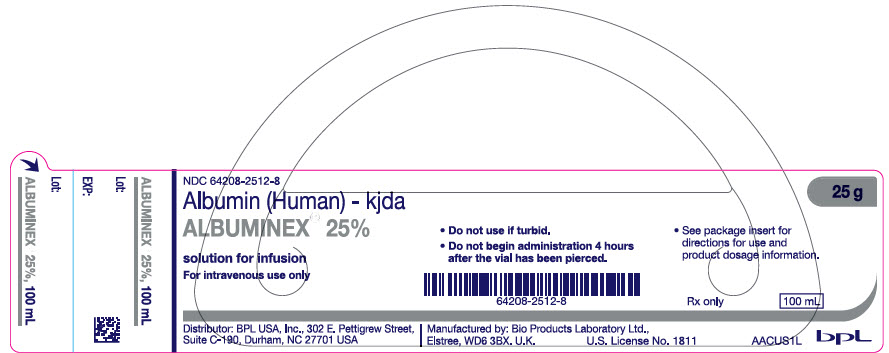
-
PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
NDC: 64208-2512-7
Albumin (Human) - kjda
ALBUMINEX® 25%
25 g
solution for infusion
For intravenous use only
Rx only
1 vial
100 mL
Bio Products Laboratory

-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
NDC: 64208-2512-4
Albumin (Human) - kjda
ALBUMINEX® 25%
solution for infusion
For intravenous use onlyDistributor: BPL USA, Inc., 302 E. Pettigrew Street,
Suite C-190, Durham, NC 27701 USA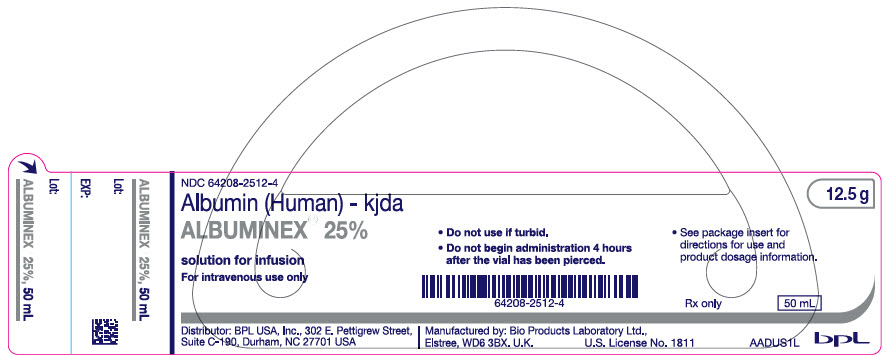
-
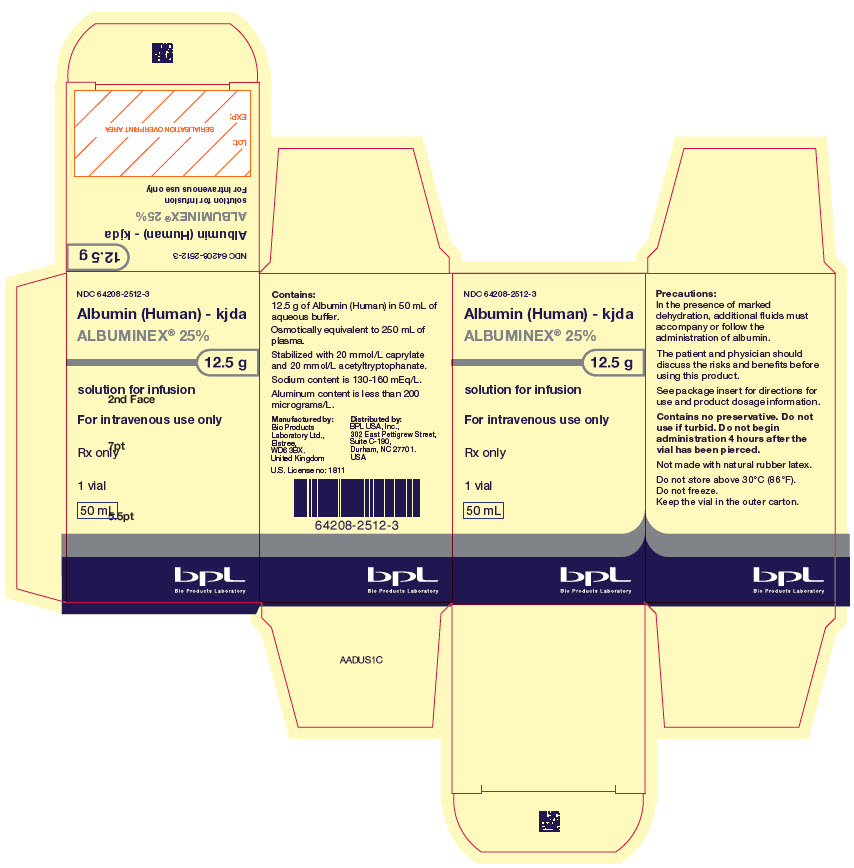
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC: 64208-2512-3
Albumin (Human) - kjda
ALBUMINEX® 25%
12.5 g
solution for infusion
For intravenous use only
Rx only
1 vial
50 mL
Bio Products Laboratory
-
INGREDIENTS AND APPEARANCE
ALBUMINEX
albumin human solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64208-2512 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albumin Human (UNII: ZIF514RVZR) (Albumin Human - UNII:ZIF514RVZR) Albumin Human 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Caprylic Acid (UNII: OBL58JN025) Sodium acetyltryptophanate (UNII: 3EN9H0M2FX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64208-2512-7 1 in 1 CARTON 02/01/2020 1 NDC: 64208-2512-8 100 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 64208-2512-3 1 in 1 CARTON 02/01/2020 2 NDC: 64208-2512-4 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125644 02/01/2020 Labeler - BPL (216845337)
Trademark Results [ALBUMINEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALBUMINEX 86927706 5751609 Live/Registered |
Bio Products Laboratory Limited 2016-03-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.